Situation at a glance
Description of the situation
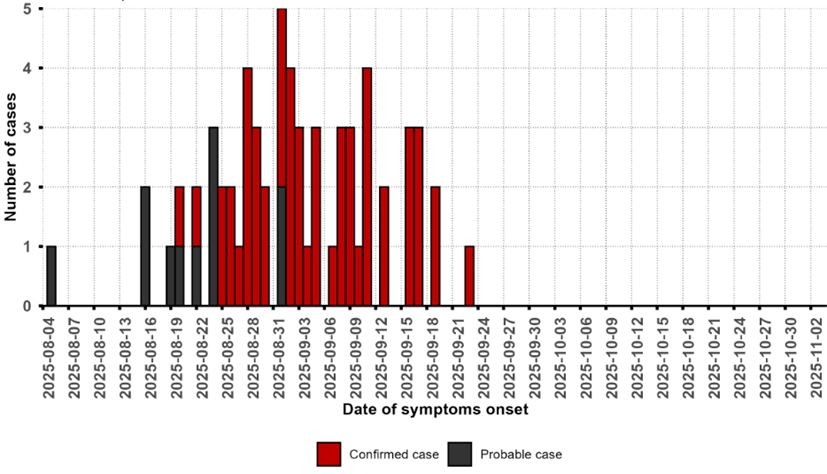
The EVD outbreak in the Democratic Republic of the Congo (DRC) was declared on 4 September 2025. As of 30 November 2025, a total of 64 cases (53 confirmed, 11 probable), including 45 deaths (CFR 70.3%), have been reported from six health areas (Bambalaie, Bulape, Bulape Com, Dikolo, Ingongo and Mpianga) in Bulape Health Zone, Kasai Province. Since the last confirmed case reported on 25 September 2025, no new confirmed EVD cases have been reported.
There have been five cases among health workers (four nurses and one laboratory technician), three of whom have died. The epicentres of the outbreak have been localised in Dikolo (26 cases, 15 deaths) and Bulape (24 cases, 22 deaths) health areas, which together account for 78.1% of the total cases reported and 82.2% of all deaths. The outbreak initially involved nosocomial transmission and a high-transmission funeral gathering, with high mortality among young children. As of 12 October 2025, a total of 572 contacts were followed up.
On 1 December 2025, the Ministry of Health declared the end of the outbreak. This declaration came after two consecutive incubation periods (42 days) since the last person confirmed with EVD tested negative for the virus and was discharged on 19 October 2025, as per WHO recommendations.
Figure 1. Map of confirmed and probable cases and deaths of Ebola virus disease, Bulape Health Zone, Kasai province, Democratic Republic of the Congo, as of 30 November 2025
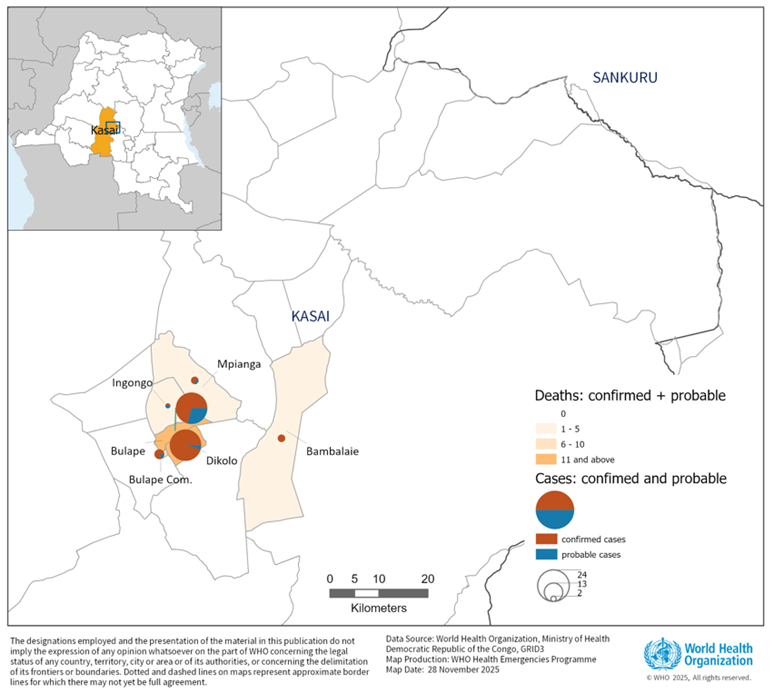
Figure 2: Epidemic curve of confirmed and probable Ebola virus disease cases in Bulape Health Zone, Kasai province, Democratic Republic of the Congo, as of 30 November 2025
Epidemiology
Ebola virus disease is a severe disease caused by the Ebola virus (EBOV). The virus is transmitted to humans through close contact with the blood or secretions of infected wildlife and then spreads through human-to-human transmission by direct contact with bodily fluids, organs, or contaminated surfaces and materials.
The incubation period, the time between infection with the virus and the onset of symptoms, ranges from 2 to 21 days, but typically is 7–11 days. People are not infectious during the incubation period; they become contagious with early symptoms; therefore, transmission risk begins at the onset of clinical signs and increases with disease severity.
Case fatality ratios ranging from 25% to 90% have been reported in previous outbreaks. The disease is characterized by an acute onset of fever with non-specific symptoms/signs (e.g., abdominal pain, anorexia, fatigue, malaise, myalgia, sore throat) usually followed several days later by nausea, vomiting, diarrhoea, and occasionally a variable rash. Severe illness may include haemorrhagic manifestations (e.g., bleeding), encephalopathy, shock/hypotension, multi-organ failure, and spontaneous abortion in infected pregnant women. Individuals who recover may experience prolonged sequelae (e.g., arthralgia, neurocognitive dysfunction, uveitis, sometimes followed by cataract formation), and clinical and subclinical persistent infection may occur in immune-privileged compartments (e.g., central nervous system, eyes, testes). Family members, health and care providers, and participants in burial ceremonies with direct contact with the deceased are at particular risk.
Public health response
Health authorities, with support from WHO and partners, implemented public health measures, including but not limited to the following:
Coordination
- The Ministry of Health (MoH) coordinated the outbreak response with WHO and partners, while the Incident Management Team in Bulape Health Zone oversaw field operations.
- A high-level national delegation led by the Minister of Health visited Kasai Province to assess response activities, reaffirm government commitment, and inaugurate a newly constructed Ebola Treatment Centre.
- WHO deployed 112 experts and frontline responders to support the national authorities to swiftly scale up and sustain the response.
- A regional strategic preparedness and response plan was developed and disseminated to guide efforts in surveillance, case management, diagnostics, vaccination, IPC, community engagement, and operational readiness.
- WHO launched a US$21 million appeal to scale up response operations, supported by contributions from partners.
Surveillance
- Surveillance activities were scaled up in Bulape and nearby areas, and more than 100 alerts were investigated.
- Community health workers were trained to support community-based surveillance using simplified case definitions.
- Congolese Red Cross volunteers were engaged in reporting community deaths and supporting surveillance efforts, while mortality surveillance was initiated in health facilities
- Surveillance, health screening and risk communication were reinforced at points of entry and points of control, including border crossings, with sensitization of staff at points of entry to detect and manage suspected cases.
- Border communities were integrated into early warning systems and the national surveillance network.
- WHO deployed epidemiologists in Bulape and supports the 90-day heightened surveillance period following the declaration of the end of the outbreak.
Laboratory
- MoH and partners strengthened laboratory capacities and deployed a mobile laboratory to reduce turnaround time for laboratory results.
- MoH performed full genome sequencing on the sample of the first confirmed case and findings indicate the outbreak was most likely the result of a spillover event from a zoonotic reservoir.
Case management
- MoH, with support from WHO and partners, set up an Ebola treatment centre in Bulape
- Case management strategy was scaled up to ensure sufficient capacities to provide care for all probable and confirmed cases in all hotspots.
- Surge teams and partners supported clinical care. Patients received monoclonal antibody treatment.
- WHO experts supported case management, essential health services, and survivor program implementation.
Vaccination
- A total of 47 577 individuals were vaccinated with the rVSVΔG-ZEBOV-GP (Ervebo) Ebola vaccine in Bulape, Bulambae, and Mweka Health Zones.
- A ring vaccination strategy implemented, targeting contacts, potential contacts, and high-risk healthcare/frontline workers, complemented by geographic targeting in hotspots.
- Ultra cold chain equipment installed in Kananga, Mweka and Tshikapa to support vaccine storage and distribution.
Infection prevention and control
- Infection prevention and control (IPC) response coordination mechanism activated, including the IPC ring around cases, which included cleaning and disinfection of sites where confirmed cases passed through.
- Recommendations provided to health workers, district leaders, and the public to strengthen detection of suspected cases and implement appropriate IPC measures.
- Supervision and support provided to Bulape General Hospital, Ebola treatment centre , and four health centres.
Risk Communication and Community Engagement
- An integrated community engagement approach was implemented, enabling the Risk Communication and Community Engagement (RCCE) team to work alongside other response pillars to facilitate safe access to affected communities and strengthen acceptance of response activities such as community surveillance, contact tracing and vaccination.
- Tailored risk communication messages were developed and disseminated widely, promoting protective behaviours and timely care- seeking, while sustained and evolving engagement with religious leaders, teachers, traditional healers and other trusted influencers helped build trust and community cooperation.
- WHO provided technical guidance and on the ground expertise to conduct a rapid community assessment to better understand the knowledge, perceptions, experiences, needs and bottom-up solutions of local communities affected by the EVD outbreak. These findings are being used to inform appropriate and localized public health measures for community protection. Community health volunteers were trained and supported, expanding local capacity for community outreach and engagement.
Operations Support and Logistics
- WHO and partners established a temporary airbridge to accelerate delivery of supplies and personnel to affected areas.
- WHO delivered over 150 tonnes of medical supplies and equipment to protect health workers and communities. Additional logistics include an epi-shuttle, generator, motorbikes, mattresses, and food for patients.
- The coordination with partners enabled rapid access to remote health zones.
Preparedness and readiness
- Eight of nine neighbouring countries completed readiness assessments
- DRC’s high-risk provinces were supported in planning.
- Capacity building conducted on readiness in five health zones
Prevention of Sexual Exploitation, Abuse and Harassment
- Prevention of sexual exploitation, abuse, and harassment (PRSEAH) integrated into response activities through responder briefings, community sensitization, and risk analysis.
- Individuals from three churches and responders/community members were oriented on PRSEAH and reporting mechanisms.
- PRSEAH focal points identified in collaboration with the Bulape Health Zone authorities.
- Posters with PRSEAH principles and reporting information were displayed in health centres and offices.
WHO risk assessment
The current outbreak constitutes the 16th Ebola disease occurrence in the DRC since 1976. The last outbreak was reported from North Kivu in 2022.
This outbreak occurred in difficult, hard to reach areas with limited existing infrastructure. A future outbreak is not unexpected given that EVD is endemic in the country. Ebola virus is enzootic and a resurgence from viral persistence in survivors has been described in recent epidemics. Re-emergence of EVD is a major public health concern in the Democratic Republic of the Congo; gaps remain in the country's capacity to recover, prepare for, and respond to outbreaks. The country is facing several outbreaks, including mpox, cholera, and measles. In addition, the country is experiencing a long-term economic and political crisis. The country's resources and capacity to effectively respond to the outbreak were therefore limited.
The epicentre of the outbreak was in proximity with the Angolan border (approximately 100 to 200 kilometres, depending on the nearest border crossing point). Although the affected district is a hard-to-reach rural area relatively far from the two main urban centres of Mbuji Mayi and Kananga, population movements between different parts of the province are frequent, especially between Bulape and Tshikapa.
The outbreak has most likely originated from a new zoonotic spillover and led to sustained human-to-human transmission. Infections and deaths among healthcare workers were reported, which raised the risk of nosocomial amplification and further spread within health facilities.
The outbreak is declared over, as of 30 November 2025, with no new cases reported for 42 consecutive days.
WHO advice
Coordination
- Outbreak control requires a coordinated, multi-sectoral approach.
- Key interventions include clinical care, surveillance, laboratory services, IPC/WASH, safe burials, and community engagement.
- Collaboration with neighbouring countries is essential for joint investigations, harmonized reporting, and real-time data sharing.
- Maintaining collaborative relationships with survivor associations while monitoring survivors is a priority to mitigate any potential risks.
Surveillance and Laboratory
- Surveillance must be strengthened at community level, health facilities, and points of entry/points of crossing.
- Suspected cases should be promptly identified, tested, and isolated.
- Laboratory capacity must support timely diagnosis and confirmation of cases.
- Contact tracing and monitoring of survivors are essential to prevent further transmission.
Case Management
- To reduce EVD mortality, early diagnosis and initiation of supportive care are essential.
- WHO-recommended treatments include Inmazeb® (3-antibody combination) and Ebanga® (single antibody).
- Ebola treatment centres must ensure biosecurity, IPC, and allow direct patient observation in red zones. WHO and partners’ innovative solutions should be utilized to ensure safe and effective care delivery.
Vaccination
- The Ervebo vaccine is recommended for ring vaccination during EVD outbreaks caused by EBOV.
- Target groups include contacts, potential contacts of confirmed/suspected cases, and frontline workers.
Risk Communication and Community Engagement (RCCE)
- Enhanced RCCE interventions should be maintained where possible to ensure communities know the signs of Ebola, understand the importance of reporting symptoms quickly and remain aware of available health services. Community engagement and feedback systems should be maintained to quickly detect concerns, rumours or changes in community perceptions that could signal any emerging risk or resurgence.
- Work should continue to support stigma-reduction and survivor reintegration, working with local leaders, survivor groups, and health workers to promote positive narratives and prevent discrimination against returning patients and their families.
Infection Prevention and Control / Water, Sanitation and Hygiene (IPC/WASH)
- Health workers caring for patients with confirmed or suspected Ebola should apply transmission-based precautions in addition to standard precautions, including PPE as per WHO's Infection prevention and control guideline for Ebola and Marburg disease and hand hygiene according to the WHO 5 moments.
- National guidelines should be followed on rules and regulations for safe waste disposal or WHO’s guidelines on safe waste management
- Healthcare facilities should maintain clean environments with safe water, sanitation, and hygiene infrastructure as outlined in Essential environmental health standards in health care.
- Safe water, adequate sanitation and hygiene infrastructure and services should be provided in healthcare facilities. For details on recommendations and improvement, follow the WASH FIT implementation Package.
Preparedness and readiness
- Bordering countries should enhance readiness for early detection, isolation, and treatment.
- Strengthen surveillance, laboratory capacity, and coordination mechanisms.
- Ensure healthcare facilities are equipped, and staff are trained in IPC/WASH and case management.
WHO advises against any restrictions on travel and/or trade to the Democratic Republic of the Congo based on available information for the outbreak.
Further information
- World Health Organization. Democratic Republic of the Congo declares end of 16th Ebola outbreak. 2025. Available: https://www.afro.who.int/countries/democratic-republic-of-congo/news/democratic-republic-congo-declares-end-of16thebola-outbreak
- Democratic Republic of the Congo. Ebola virus disease outbreak declared in Kasai Province. 2025. Available: https://www.afro.who.int/countries/democratic-republic-of-congo/news/democratic-republic-congo-declares-ebola-virus-disease-outbreak-kasai-province
- World Health Organization. Ebola virus disease: Fact sheet. Geneva: WHO; 2025. Available: https://www.who.int/news-room/fact-sheets/detail/ebola-disease
- World Health Organization. Ebola and Marburg virus disease epidemics: Preparedness, alert, control, and evaluation. Geneva: WHO; 2024. Available: https://www.who.int/publications/i/item/who-hse-ped-ced-2014.05
- World Health Organization. Infection prevention and control guideline for Ebola and Marburg disease. Geneva: WHO; 2023. Available: https://www.who.int/publications/i/item/WHO-WPE-CRS-HCR-2023.1
- World Health Organization. IPC measures for Ebola and Marburg: Past and present. Geneva: WHO; 2024. Available: https://www.who.int/multi-media/details/ipc-measures-for-ebola-and-marburg-disease--past-and-present
- World Health Organization. WHO-Strategic Research Agenda for Filovirus Research and Monitoring (WHO-AFIRM). Geneva: WHO; 2022. Available: https://www.who.int/publications/m/item/a-who-strategic-research-agenda-for-filovirus-research-and-monitoring-----(who-afirm)
- World Health Organization. Ebola and Marburg disease outbreaks: IPC research priorities in health care settings. Geneva: WHO; 2024. Available: https://www.who.int/publications/i/item/9789240098381
- Summary of WHO infection prevention and control guideline for Ebola and Marburg disease: A call for evidence-based practice. BMJ; 2024.Available: https://www.bmj.com/content/384/bmj.p2811
- Raphael G Frankfurter, Victoria Willet, Eugene T Richardson, et el. Infection prevention and control studies for care of patients with suspected or confirmed filovirus disease in healthcare settings: An integrative review. BMJ public health; 2024. Available: https://pubmed.ncbi.nlm.nih.gov/39015119/
- World Health Organization. Steps to put on PPE for Ebola/Marburg disease: Gown and headcover. Geneva: WHO; 2024. Available: https://www.who.int/multi-media/details/steps-to-put-on-ppe-for-ebola-marburg-disease-gown-and-headcover
- World Health Organization. Steps to remove PPE for Ebola/Marburg disease: Gown and headcover. Geneva: WHO; 2024. Available: https://www.who.int/multi-media/details/steps-to-remove-ppe-for-ebola-marburg-disease-gown-and-headcover
- World Health Organization. Steps to put on PPE for Ebola/Marburg disease: Coverall. Geneva: WHO; 2024. Available: https://www.who.int/multi-media/details/steps-to-put-on-ppe-for-ebola-marburg-disease-coverall
- World Health Organization. Steps to remove PPE for Ebola/Marburg disease: Coverall. Geneva: WHO; 2024. Available: https://www.who.int/multi-media/details/steps-to-remove-ppe-for-ebola-marburg-disease-coverall
- World Health Organization. CORE trial protocol for candidate therapeutics against Ebola disease. Geneva: WHO; 2024. Available: https://www.who.int/publications/m/item/solidarity-partners-platform-adaptive-randomized-trial-for-new-and-repurpose-filovirus-treatments-core-trial-protocol
- World Health Organization. CORE trial protocol for candidate vaccines against Ebola disease. Geneva: WHO; 2024. Available: https://www.who.int/publications/m/item/core-protocol-a-phase-1-2-3-study-to-evaluate-the-safety-tolerability-immunogenicity-and-efficacy-of-vaccine-candidates-against-filoviruses-disease-in-healthy-individuals-at-risk-of-filovirus-disease
- World Health Organization. Filoviridae: Landscape of vaccines and therapeutics licensed or under development. Geneva: WHO; 2024. Available: https://www.who.int/publications/m/item/filoviridae---landscape-of-vaccines-and-therapeutics-licensed-or-under-development-for-pathogens-being-considered-as-priority-pathogens
- World Health Organization. Considerations for border health and points of entry for filovirus disease outbreaks. Geneva: WHO; 2024. Available: https://www.who.int/publications/m/item/considerations-for-border-health-and-points-of-entry-for-filovirus-disease-outbreaks
- World Health Organization. Syndromic entry and exit screening for epidemic-prone diseases of travellers at ground crossings: Systematic review. Geneva: WHO; 2024. Available: https://www.who.int/publications/i/item/9789240090309
- World Health Organization. Ebola disease event management at points of entry. Geneva: WHO; 2014. Available: https://iris.who.int/server/api/core/bitstreams/00727579-5fcf-4ab3-937c-45adf800c86a/content
- World Health Organization. Entry screening for Ebola disease at airports, ports and land crossings: Technical note for preparedness planning. Geneva: WHO; 2024. Available: https://www.who.int/publications/i/item/WHO-EVD-Guidance-PoE-14.3#:~:text=Overview.%20WHO%20does%20not%20recommend%20entry%20screening%20for
- World Health Organization. Exit screening at airports, ports and land crossings: Interim guidance for Ebola disease. Geneva: WHO; 2014. Available: https://iris.who.int/server/api/core/bitstreams/1a2e19e1-ee76-47a1-8561-6c337f273418/content
- World Health Organization. Risk communication and community engagement readiness and response toolkit: Ebola disease. Geneva: WHO; 2025. Available: https://www.who.int/publications/i/item/9789240110175
- World Health Organization. Diagnostic testing for Ebola and Marburg diseases: Interim guidance. Geneva: WHO; 2024. Available: Diagnostic testing for Ebola and Marburg virus diseases: interim guidance, 20 December 2024
- World Health Organization. How to safely collect blood samples by phlebotomy from patients suspected to be infected with filovirus. Geneva: WHO; 2014. Available: https://www.who.int/publications/i/item/WHO-EVD-Guidance-Lab-14.4
- World Health Organization. How to safely collect oral swabs (saliva) from deceased patients suspected to be infected with filovirus. Geneva: WHO; 2014. Available: https://www.who.int/publications/i/item/how-to-safely-collect-oral-swabs-from-deceased-patients-suspected-to-be-infected-with-ebola-or-marburg
- World Health Organization. How to safely ship human blood samples from suspected EBOD cases within a country by road, rail and sea. Geneva: WHO; 2017. Available: https://www.who.int/publications/i/item/how-to-safely-ship-human-blood-samples-from-suspected-ebola-or-marburg-cases-within-a-country-by-road-rail-and-sea
- World Health Organization. Optimized supportive care for Ebola virus disease: Clinical management SOPs. Geneva: WHO; 2019. Available: https://www.who.int/publications/i/item/9789241515894#:s
- World Health Organization. ICD-11 release. Geneva: WHO; 2022. Available: https://www.who.int/news/item/11-02-2022-icd-11-2022-release
- Kuhn JH, Adachi T, Adhikari NKJ, et al. New filovirus disease classification and nomenclature. Nature Reviews Microbiology. 2019. Available: https://pmc.ncbi.nlm.nih.gov/articles/PMC6637750/#SD1
- World Health Organization. Diagnostic testing for Ebola and Marburg virus diseases. Geneva: WHO; 2025. Available: https://www.who.int/publications/i/item/B09221
- World Health Organization. WHO R&D Blueprint for Epidemics and Filoviruses. Geneva: WHO; 2025. Available: https://www.who.int/teams/blueprint/ebolavirus
- World Health Organization. Pathogens prioritization: A scientific framework for epidemic and pandemic research preparedness. Geneva: WHO; 2024. Available: https://www.who.int/publications/m/item/pathogens-prioritization-a-scientific-framework-for-epidemic-and-pandemic-research-preparedness
- World Health Organization. ICG Ebola vaccine stockpile. Geneva: WHO; 2025. Available: https://www.who.int/groups/icg/ebola-virus-disease
- World Health Organization. WHO launches online training to strengthen filovirus outbreak response. Geneva: WHO; 2025. Available: https://www.who.int/news/item/26-03-2025-who-launches-online-training-to-strengthen-filovirus-outbreak-response#
Citable reference: World Health Organization (1 December 2025). Disease Outbreak News; Ebola virus disease in the Democratic Republic of the Congo. Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2025-DON589
