Since July 2020, WHO and the Ministry of Health are carrying out a seroepidemiological study to understand how and to what extent the population across Indonesia has been infected with SARS-CoV-2, and assess the proportion of people with antibodies against SARS-CoV-2 in different genders and age groups in general population.
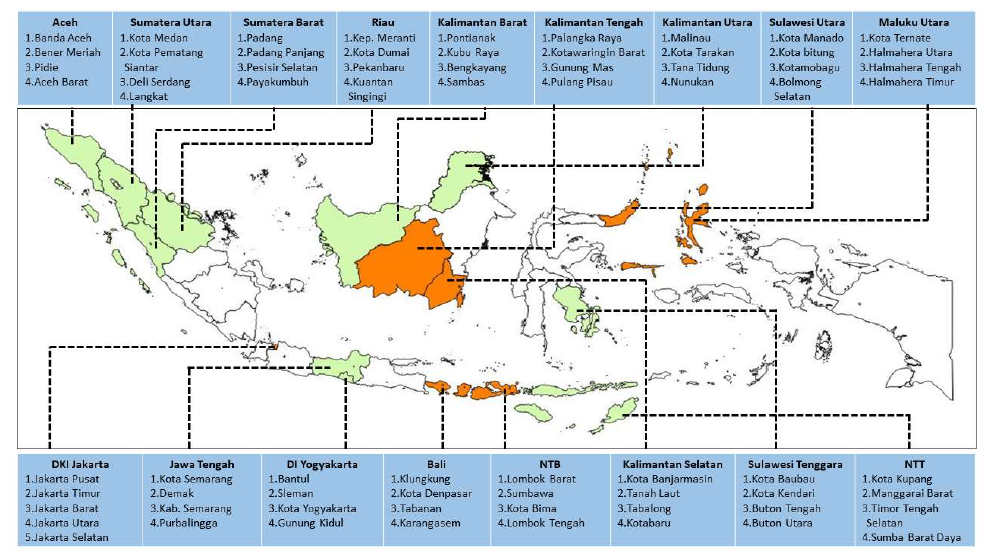
Caption: A map of the provinces and districts across Indonesia participating in the nationwide seroepidemiological study
Image Credit: WHO
10 200 individuals from seven high-burden provinces and ten low-burden provinces based on August 2020 data have participated in this study. To date, data collection has been completed from all districts, except one district in South Kalimantan Province that was affected by flooding. WHO is in the process of facilitating the transport of samples from the study sites to six selected laboratories for Enzyme Linked Immunosorbent Assay (ELISA) examination and to the NIHRD laboratory for quality control. The results of this survey are expected by the end of March 2021. Indonesia’s participation in the seroepidemiological study will contribute not only to the national public health response and policy decisions, but also to the global understanding of seroprevalence levels and control measures.
Caption: WHO and USAID delivered external quality assurance (EQA) panels to COVID-19 testing laboratories across Indonesia.
Image Credit: The National Institute of Health Research and Development (NIHRD)
A quality management mechanism is essential to ensure that COVID‑19 laboratories provide accurate and reliable results; this includes proficiency testing or external quality assurance (EQA). WHO and the USAID Infectious Disease Detection and Surveillance have supported NIHRD to distribute proficiency test panels to 177 laboratories across Indonesia. By the end of January 2021, most laboratories across Indonesia have submitted the results to NIHRD for preliminary data analysis. The analysis will inform NIHRD on overall laboratory quality and areas of improvement, to be discussed in a series of evaluation meetings with the COVID-19 laboratory network across Indonesia.
As a part of the comprehensive strategy to improve COVID-19 laboratory capacity in Indonesia, WHO handed over 248 sets of magnetic stands (at an estimated cost of USD 18 600) and a high-throughput automated sample preparation system for nucleic acid extraction (about USD 150 000) to the NIHRD on 26 and 29 January 2021. The magnetic stands will be distributed to laboratories across Indonesia to be used in the manual nucleic acid extraction process. The high-throughput extraction system is expected to increase the SARS-CoV-2 testing capacity at NIHRD by reducing the required time and numbers of laboratory staff needed to conduct testing.

Caption: A training participant utilizes laboratory equipment during a practical training exercise on polymerase chain reaction (PRC) testing held by WHO
Credit: WHO
Well-functioning laboratories are the backbone of robust surveillance system. Since March 2020, WHO has provided technical assistance and capacity building support to improve and scale up laboratory capacity across the country. With WHO technical support, MoH has expanded the laboratory network for COVID-19 testing from 18 laboratories at the start of the pandemic to 612 laboratories as of 22 January 2021. More than 900 laboratory technicians have been trained in polymerase chain reaction (PCR) testing, biosafety, and security. Together with partners, WHO has supported the delivery of critical laboratory supplies and lifesaving equipment worth USD 14 million, one million antigen rapid diagnostic tests, 506 600 viral transport mediums and swabs, and PCR kits for 500 000 individual tests.
