Validation process & tools
In 2014, WHO released the first edition of the Global guidance on criteria and processes for validation: elimination of mother-to-child transmission (EMTCT) of HIV and syphilis. In 2015, the Global Validation Advisory Committee for EMTCT was established. Since then, Member States have been able to apply for, and be validated for, achieving EMTCT of HIV and/or syphilis, to a level where it is no longer a public health threat. In alignment with the framework for Triple Elimination, WHO published updated guidance in 2021 which incorporated criteria and processes for validation of EMTCT of hepatitis B virus (HBV). Now Member States are able to apply for validation of Triple Elimination of MTCT of HIV, syphilis and HBV.
WHO oversees the validation secretariat in collaboration with United Nations partners from the Joint United Nations Programme on HIV/AIDS (UNAIDS), the United Nations Children’s Fund (UNICEF), the United Nations Population Fund (UNFPA) and the International Organization for Migration (IOM). All WHO regions have established validation structures to support the process. At the global level, the Global Validation Advisory Committee (GVAC) reviews the evidence provided in validation reports and gives independent advice to WHO on whether a country has met the criteria to be validated.
Process for validation of EMTCT
Before initiating the EMTCT validation process, countries should be confident that they can meet the global minimum criteria and foundational requirements for validation.
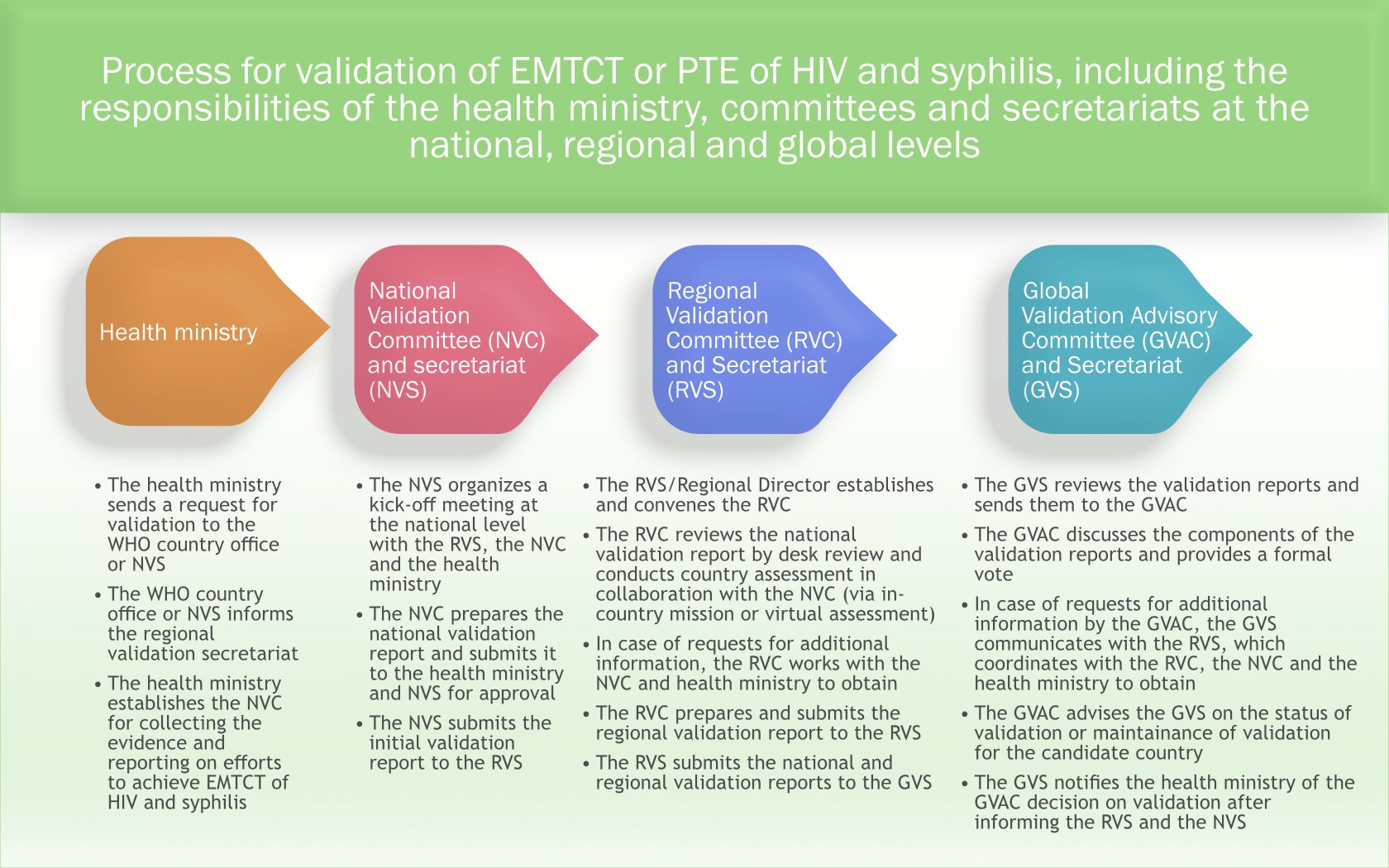
The process for validation includes responsibilities for health ministries, committees and secretariats at the global, regional and national levels, as described in the governance guidance.
Global guidance
Global guidance on criteria and processes for validation: elimination of mother-to-child...
Governance for the validation of elimination of mother-to-child transmission of HIV, syphilis and hepatitis...
Achieving validation of elimination of mother-to-child transmission (EMTCT) of HIV, syphilis and hepatitis B virus (HBV) is a tremendous accomplishment,...
Tools for validation
Tools and checklists for in-country assessment of the four required components and report templates
Web Annex A. Checklist for country preliminary assessment of EMTCT of HIV, syphilis and hepatitis B virus and Path to Elimination criteria | Spanish | Portuguese
Web Annex B. Congenital syphilis estimation tool
Web Annex C. Initial validation report on the elimination of mother-to-child transmission of HIV, syphilis and hepatitis B virus | Spanish | Portuguese
Web Annex D. Data assessment and verification tool | Spanish | Portuguese
Web Annex E. Laboratory assessment and verification tool | Spanish | Portuguese
Web Annex F. Programme assessment and verification tool | Spanish | Portuguese
Web Annex G. Human rights, gender equality and community engagement assessment and verification tool | Spanish | Portuguese
Web Annex H. Analysis guidance for human rights, gender equality and community engagement in validation | Spanish | Portuguese
Web Annex I. Sample case study form | Spanish | Portuguese
Web Annex J. Maintenance of validation of elimination of mother-to-child transmission of HIV, syphilis and hepatitis B virus | Spanish | Portuguese