WHO Health Technology Access Programme
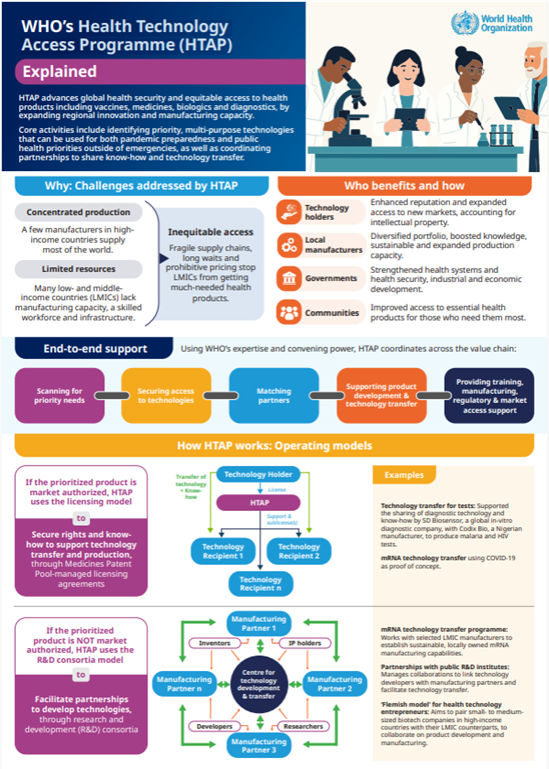
The Health Technology Access Programme (HTAP) is a WHO initiative that advances global equitable access to health products and health security by expanding regional innovation and manufacturing capacity. HTAP selects priority technologies and mobilizes partners to support their development, transfer and sustainable production to make them available for communities that need them.
Collaborative and demand-driven, HTAP aims to enhance health security, address unmet health needs and bridge the global health technology access gap. It covers both pandemic preparedness and public health priorities outside of emergencies. HTAP has initially focused on mRNA vaccines and diagnostics and will consider other innovative technologies based on public health needs and their business case.
HTAP succeeds the COVID-19 Technology Access Pool (C-TAP) and fully integrates the mRNA technology transfer programme, which were both originally established to address inequitable access to COVID-19 vaccines, medicines and other health products.
Harnessing WHO’s expertise, convening power and mandate, HTAP coordinates WHO and partners to support health product manufacturing from end to end. Through two operating models, HTAP works to foster research and development (R&D) and secure the transfer of technology and know-how for health products.
Flagship activities include:
- Facilitating technology transfer sublicensing agreements that provide manufacturers in low- and middle-income countries (LMICs) with the rights, know-how and material to manufacture rapid diagnostic testing (RDT) technology from SD Biosensor, a company headquartered in the Republic of Korea.
- The mRNA Technology Transfer Programme, which works with 15 selected manufacturers from LMICs to improve health security by establishing sustainable, locally owned mRNA manufacturing capabilities. The programme aims to establish R&D consortia focused on 8 targets (including diseases as well as components to make mRNA-based products) to develop commercially viable mRNA products that can be manufactured by participating partners. These targets address local and regional priority diseases, such as dengue and hand foot and mouth disease in South-East Asia and influenza and leishmaniasis in Latin America. The aim is to establish sustainable mRNA production, so in the case of a health emergency, such as a pandemic, it can be quickly repurposed to address the new threat.
- Partnerships with public R&D institutes, the Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST) in India, and the University of Cape Town (UCT) in South Africa. While still evolving, these collaborations are expected to expand equitable access to innovative technologies, including by linking technology developers with manufacturing partners and facilitating technology transfer.
HTAP is primarily funded by the Government of Belgium, the European Union and the Government of Spain. In-service contributions are also received from the Government of the People’s Republic of China and the Republic of France. The content presented here is the sole responsibility of WHO and does not necessarily reflect the views of the donors.