Throughout 2020 countries massively increased the number of laboratories that were testing for SARS-CoV-2 using polymerase chain reaction (PCR) assays. However, many countries did not have a means to monitor the quality of testing in these laboratories, many of which had limited experience in the type of testing there were now being asked to perform.
To provide countries with a better understanding of performance of their laboratories, WHO organized a global round of laboratory proficiency testing designed to include sub-national laboratories. A total of 3,300 panels consisting of five specimens were prepared and distributed to national and sub-national laboratories. In total, 1809 laboratories reported their results back to WHO from 100 countries (52% of Member States).
Laboratories were asked to test the specimens in the panel and report their results. Participants were given the opportunity to report two sets of results if they used more than one platform to test for SARS-CoV-2. Of the 1809 laboratories that reported their results back to WHO, 775 laboratories reported two sets of results. This meant a total of 2,584 sets of results were available for assessment.
The performance of participating laboratories was very good with only 2.7% of assessed results being incorrect (see figure)
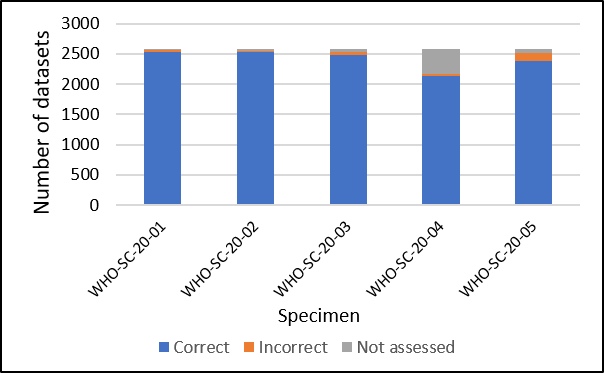
Results were not assessed if they were left blank or reported as “not tested” or “invalid”. The specimen with the most incorrect results reported was the sample which contained the lowest concentration of SARS-CoV-2 nucleic acid.
Details of the performance of laboratories were reported back to the participants and shared with the ministries of health in each country. These results will give Member States greater confidence in the results being reported by their laboratories and will assist them to target support to poorly performing laboratories.
This initiative was supported by The European Commission through its CBRN Centers of Excellence Initiative
