This Medical Product Alert relates to four confirmed falsified products: quinine sulphate circulating in Central African Republic and Chad and quinine bisulphate circulating in Uganda. It should be noted that this is the second WHO Medical Product Alert issued on the same batch number of falsified quinine bisulphate circulating in the African region. Please refer to the previous WHO Medical Product Alert N°2/2017 issued 27 July 2017. Quinine bisulphate and quinine sulphate are listed on the WHO Model List of Essential Medicines for management of severe malaria. This alert compiles details of four different falsified products that have been discovered at different times and locations over the past year. This repeated occurrence highlights the need for additional vigilance in Central and East Africa for such products that display common characteristics and distribution patterns.
1. QUININE SULPHATE BP 300mg identified in the Central African Republic
WHO was informed by a non-governmental organization (NGO) that this falsified quinine sulphate was found at patient level in the Central African Republic. At this stage, testing has not yet been conducted. Product details are listed in Table 1 below.
Table 1: Details of the falsified Quinine Sulphate BP 300mg, subject of WHO medical product alert N°10/2019
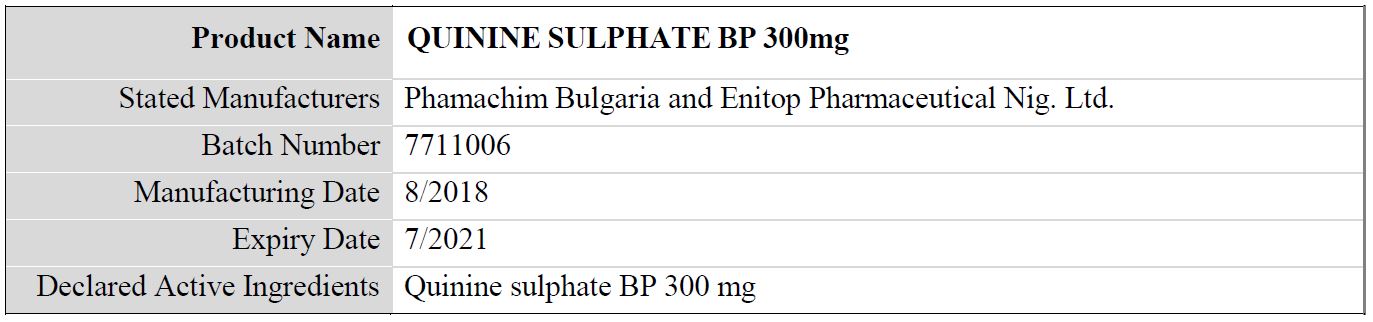
Regarding the stated manufacturer, it should be noted that:
- The packaging indicates the product to be manufactured by “Enitop Pharmaceutical Nig. Ltd.” The National Agency for Food and Drug Administration and Control (NAFDAC) in Nigeria has confirmed that “Enitop Pharmaceutical Nig. Ltd.” is not registered as a manufacturer.
- The packaging indicates the product to be manufactured by “Phamachim Bulgaria”. This manufacturer does not exist.
It should also be noted that:
- At this stage, no adverse reactions have been reported to WHO.
- The packaging is in French language but displays numerous inconsistencies.
- The registration number stated on the packaging refers to the National Agency for Food and Drug Administration and Control (NAFDAC) in Nigeria. NAFDAC has confirmed that this is falsified.
2. QUININE SULPHATE BP 800mg identified in the Central African Republic
WHO was recently informed by a non-governmental organization (NGO) that this falsified quinine sulphate was found at patient level in the Central African Republic. Preliminary testing indicated that the product did not contain any of the stated active ingredient. Product details are listed in Table 2 below.
Table 2: Details of the falsified Quinine Sulphate BP 800mg, subject of WHO medical product alert N°10/2019
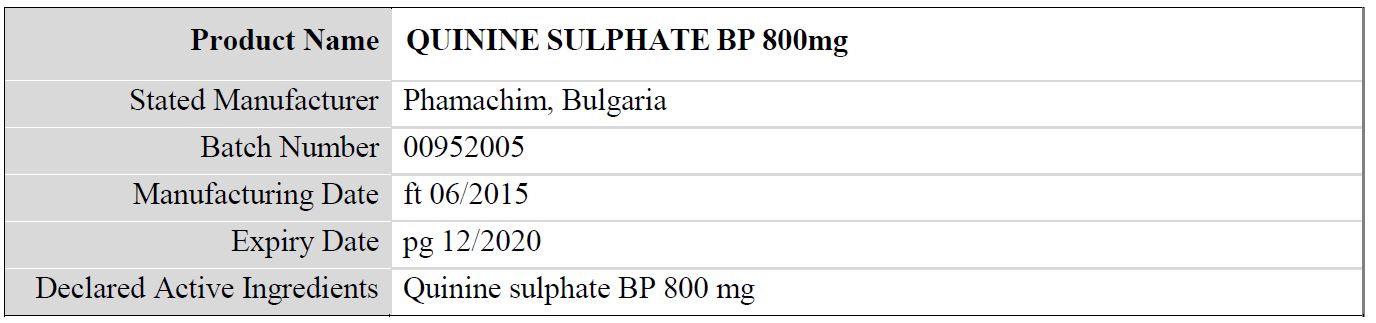
Regarding the stated manufacturer, it should be noted that:
- The packaging indicates the product to be manufactured by “Phamachim, Bulgaria”. This manufacturer does not exist.
It should also be noted that:
- Thin layer chromatography did not identify the expected active ingredient.
- At this stage, no adverse reactions have been reported to WHO.
- The packaging is in French language but displays numerous inconsistencies.
- The registration number stated on the packaging refers to the National Agency for Food and Drug Administration and Control (NAFDAC) in Nigeria. NAFDAC has confirmed that this is falsified.
3. QUININE Bisulphate 300mg. B.P. identified in Uganda
WHO was recently informed by the Uganda National Drug Authority that falsified quinine bisulphate was found at patient level in Uganda through routine post marketing surveillance on the quality of medical products in the market. Samples were sent for laboratory testing and the results were shared with WHO. Product details are listed in Table 3 below and are contained in the Uganda National Drug Authority News Release
Table 3: Details of the falsified Quinine Bisulphate 300mg. B.P., subject of WHO medical product alert N°10/2019
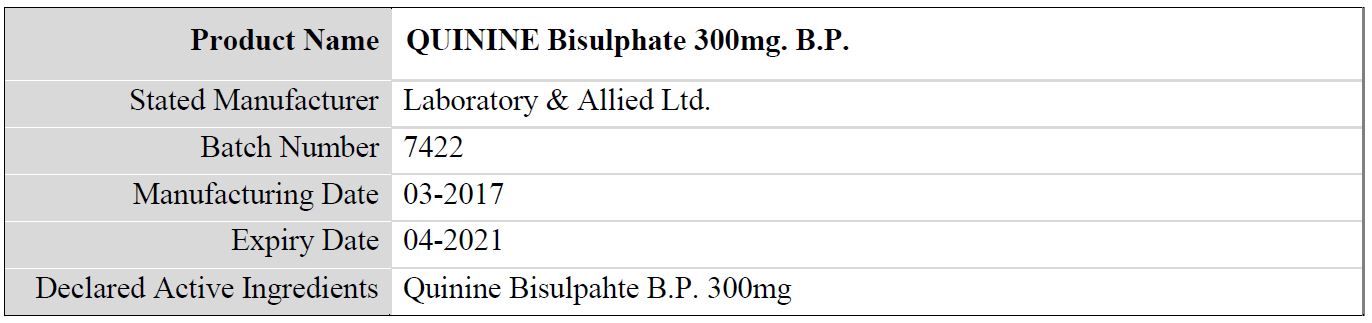
The above stated manufacturer has confirmed that:
- They did not manufacture this falsified version.
- The variable details on the product label do not correspond to the genuine manufacturer records.
It should also be noted that:
- Laboratory analysis did not identify the expected active ingredient.
- At this stage, no adverse reactions have been reported to WHO.
- There are labelling and packaging inconsistencies, including spelling errors.
4. QUININE SULPHATE BP 300mg identified in Chad
WHO was informed by a non-governmental organization (NGO) that this falsified quinine sulphate was found at patient level in Chad. Preliminary testing indicated that the product did not contain any of the stated active ingredient. Product details are listed in Table 4 below.
Table 4: Details of the falsified Quinine Sulphate BP 300mg, subject of WHO medical product alert N°10/2019
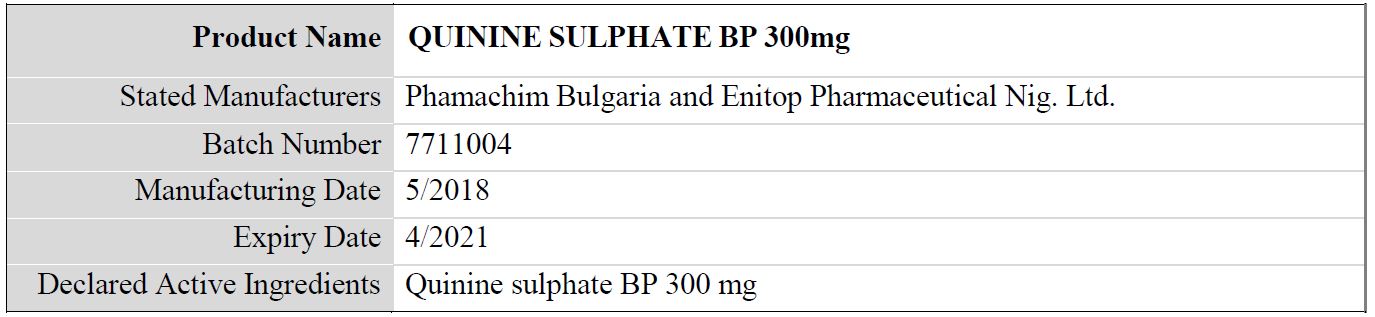
Regarding the stated manufacturer, it should be noted that:
- The packaging indicates the product to be manufactured by “Enitop Pharmaceutical Nig. Ltd. The National Agency for Food and Drug Administration and Control (NAFDAC) in Nigeria has confirmed that “Enitop Pharmaceutical Nig. Ltd.” is not registered as a manufacturer.
- The packaging indicates the product to be manufactured by “Phamachim Bulgaria”. This manufacturer does not exist.
It should also be noted that:
- Thin layer chromatography did not identify the expected active ingredient.
- At this stage, no adverse reactions have been reported to WHO.
- The packaging is in French language but displays numerous inconsistencies.
- The registration number stated on the packaging refers to the National Agency for Food and Drug Administration and Control (NAFDAC) in Nigeria. NAFDAC has confirmed that this is falsified.
Photographs and advice to the public are available below.
QUININE SULPHATE BP 300mg, batch 7711006 identified in the Central African Republic

QUININE SULPHATE BP 800mg, batch number 00952005 identified in the Central African Republic

QUININE Bisulphate 300mg. B.P., batch number 7422 identified in Uganda

QUININE SULPHATE BP 300mg, batch 7711004 identified in Chad

WHO requests increased vigilance within the supply chains of countries likely to be affected by these falsified medical products. Increased vigilance should include hospitals, clinics, health centres, wholesalers, distributors, pharmacies and any other suppliers of medical products.
If you are in possession of the above specific products, please do not use. If you have taken these falsified medical products, or if you suffer an adverse event or an unexpected lack of efficacy, please seek immediate advice from a qualified healthcare professional, and ensure they report the incident to your local Ministry of Health/National Medicines Regulatory Authorities/National Pharmacovigilance Centre.
All medical products must be obtained from authentic and reliable sources. Their authenticity and condition should be carefully checked. Seek advice from a healthcare professional in case of doubt.
National health authorities are asked to immediately notify WHO if these falsified medical products are discovered in their country. If you have any information concerning the manufacture, distribution, or supply of these medical products please contact rapidalert@who.int
WHO Global Surveillance and Monitoring Systemfor Substandard and Falsified Medical Products
For further information, please visit: http://www.who.int/medicines/regulation/ssffc/en/
