Alert Summary
This WHO Medical Product Alert refers to one batch of falsified Combiart (combination of artemether and lumefantrine) identified in Chad, Côte d'Ivoire, and Mali and reported to WHO in November 2021. The genuine manufacturer of Combiart has confirmed that the product listed in this alert is falsified. The falsified product was reported at the patient level outside authorized and regulated supply chains in the above-mentioned countries.
Genuine Combiart is indicated for the treatment of acute, uncomplicated malaria infections due to Plasmodium falciparum, and is effective in geographical regions where resistance to chloroquine has been reported.
The products identified in this alert are confirmed as falsified on the basis that they deliberately/fraudulently misrepresent their identity, composition, or source. Laboratory analyses of the products found were conducted and the two expected active ingredients (artemether and lumefantrine) were not detected.
Distinguishing features of the falsification:
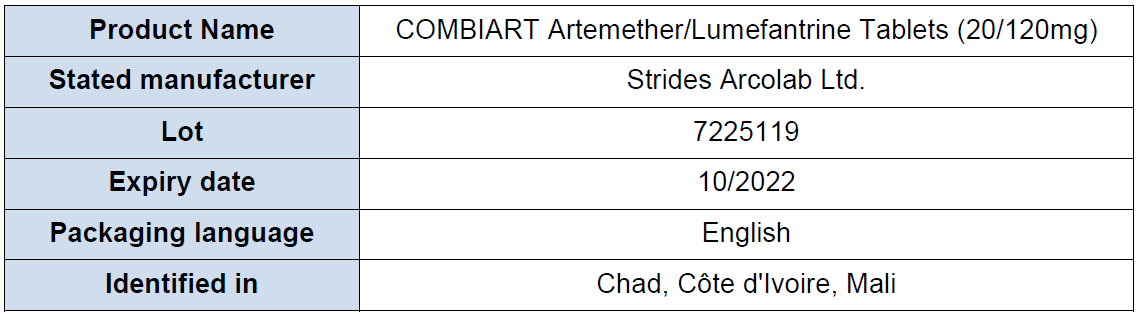
- The expiry date on the packaging is 10/2021, while the expiry date on the blister is 10/2022
- The falsified product has a Tanzania Reg No TZ13H260 on the blister
Table 1: Products subject of WHO Medical Product Alert N°8/2021
Advice to regulatory authorities and the public
WHO requests increased attention within the supply chains of countries and regions likely to be affected by these falsified products due to prevalence of malaria caused by Plasmodium falciparum. Increased surveillance should include hospitals, clinics, health centres, wholesalers, distributors, pharmacies, and any other suppliers of medical products.
All medical products must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked. Seek advice from a healthcare professional in case of doubt.
If you are in possession of the above-falsified products, please do not use them.
If you have used these products, or you suffered an adverse reaction/event having used these products, you are advised to seek immediate medical advice from a qualified healthcare professional and to report the incident to the National Regulatory Authorities/National Pharmacovigilance Centre.
National regulatory/health authorities are advised to immediately notify WHO if these falsified products are discovered in their country. If you have any information concerning the manufacture, distribution, or supply of these products, please contact rapidalert@who.int
Table 2: Photographs of products subject of WHO Medical Product Alert N°8/2021
Falsified Combiart, Lot 7225119 identified in Chad, Côte d'Ivoire and Mali

WHO Global Surveillance and Monitoring System for Substandard and Falsified Medical Products
For more information, please visit our website
Email: rapidalert@who.int
