This Medical Product Alert relates to two falsified products circulating in Iran and Pakistan, and which claim to contain meglumine antimoniate for the treatment of leishmaniosis. Both falsified products are presented in clear glass ampoules and falsely claim to be manufactured for Tillotts Pharma AG. Circulation of these falsified medical products is confirmed in the WHO Region of the Eastern Mediterranean.
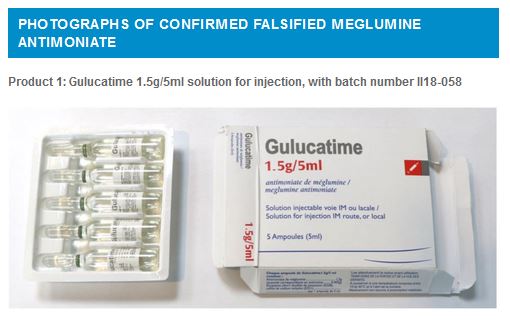
Falsified product n°1: GULUCATIME In January 2019, WHO was informed that a product called GULUCATIME was available at patient level in Iran. This product claims to be manufactured for Tillotts Pharma AG; verification by WHO confirmed that the product is falsified. It is presented in carton packs each containing five ampoules. The packing is in English and French languages but displays spelling mistakes in both languages. Product details are listed in Table 1 below.
Falsified product n°2: GLUCANTIME In March 2019, WHO was informed that a similar product called GLUCANTIME was available at patient level in Pakistan. This product also claims to be manufactured for Tillots Pharma AG and is confirmed falsified. Available photographs suggest the label is in English language only. Product details are listed in Table 1 below.
Table 1: Details of the falsified products GULUCATIME and GLUCANTIME, subject of WHO medical product alert N°7/2019

The results of laboratory analysis facilitated by WHO indicate that the product GULUCATIME was not manufactured in accordance to good manufacturing practices.
Laboratory analysis of the product GLUCANTIME is pending This medical product alert N°7/2019 will subsequently be updated and posted on the WHO website once results are known.
There have been no known adverse reactions reported to WHO at this stage attributed to the use of either of the two above-mentioned falsified products.
The packaging or labels of the two above-referenced products (Gulucatime and Glucantime) indicate “manufactured for Tillotts Pharma AG”. However, the company Tillotts Pharma AG has confirmed to WHO that they do not manufacture, sub-contract the manufacture, nor distribute these products anywhere in the world.

WHO requests increased vigilance within the supply chains of countries likely to be affected by these falsified medical products. Increased vigilance should include hospitals, clinics, health centres, wholesalers, distributors, pharmacies and any other suppliers of medical products.
If you are in possession of the above batches, please do not use. If you have taken these falsified medical products, or if you suffer an adverse event or an unexpected lack of efficacy, please seek immediate advice from a qualified healthcare professional, and ensure they report the incident to your local Ministry of Health/National Medicines Regulatory Authorities/National Pharmacovigilance Centre.
All medical products must be obtained from authentic and reliable sources. Their authenticity and condition should be carefully checked. Seek advice from a healthcare professional in case of doubt.
National health authorities are asked to immediately notify WHO if these falsified medical products are discovered in their country. If you have any information concerning the manufacture, distribution, or supply of these medical products please contact rapidalert@who.int
WHO Global Surveillance and Monitoring Systemfor Substandard and Falsified Medical Products
For further information, please visit: http://www.who.int/medicines/regulation/ssffc/en/
