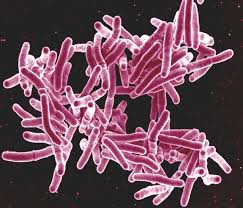
The World Health Organization welcomes the new licensing agreement between the Medicines Patent Pool (MPP) and Johns Hopkins University to facilitate the clinical development of the TB drug candidate sutezolid.
Sutezolid is an oxazolidinone antibiotic in the same class as the commercially-available drug linezolid - [used in multidrug-resistant TB (MDR-TB) treatment regimens]. Phase I trials revealed that the compound has action mechanisms similar to those of linezolid but is more potent and less toxic. While sutezolid reached Phase IIa clinical development, there have been no advances since 2013.
“The MPP-John Hopkins University agreement is an extraordinary step as it seeks to jump-start currently stalled development on a compound that showed promise in early stage trials,” said Mario Raviglione, Director of the Global TB Programme at the World Health Organization (WHO). “The current scarcity of treatment options is threatening to derail the WHO’s global targets to slash TB deaths by 90% by 2030. We are in urgent need of new and better combination regimens, especially for patients with MDR-TB, and the inclusion of sutezolid might bring great benefit.
“Sutezolid has been considered a promising investigational treatment that, if further developed in combination with other drugs, could be used to more effectively treat both drug-sensitive and drug-resistant TB in patients,” added Dr Christian Lienhardt, Team Leader, Research for TB Elimination, WHO Global TB Programme. "We commend the Medicines Patent Pool for its new commitment to facilitate treatment options for TB patients."
