Our work
The World Health Organization (WHO) has set up a Secretariat to coordinate the implementation of the World Health Assembly resolution on strengthening clinical trials (WHA75.8). The Secretariat works with Member States and partners to improve quality, transparency, and efficiency of clinical trials globally.
It provides technical support, convenes stakeholders, promotes best practices and helps strengthen clinical trial capacity so that clinical trials generate reliable evidence and meet high ethical and scientific standards. Additionally, the Secretariat monitors progress, supports the dissemination of findings, and advocates for policy reforms to optimize clinical trial processes in alignment with global health priorities.
What the Secretariat does:
- consults with Member States and non-State actors in a transparent manner to ensure inclusivity in reforming clinical trials;
- coordinates and convenes global engagement of all stakeholders in clinical trials;
- coordinates review of existing guidance to develop new guidance on clinical trials; and
- provides technical support to Member States and non-State actors on strengthening national and regional clinical trials capabilities.
Leading global development and implementation of the new Guidance for best practices for clinical trials
In response to resolution WHA75.8 (2022), the Secretariat led the development of the Guidance for best practices for clinical trials to strengthen clinical trial ecosystems worlwide.
The Guidance provides practical recommendations to improve the quality, coordination, and efficiency of clinical trials helping ensure research is ethical, fit-for-purpose and responsive to public health priorities in diverse settings, including during emergencies.
Developed through extensive global consultation, the Guidance reflects input from a global survey and expert feedback spanning more than 48 countries, ensuring it is relevant across diverse settings and health systems.
To support the development of the guidance, WHO formed a Technical Advisory Group on Development of Guidance on Best Practices for Clinical Trials, in adherence to WHO Guidance development process.
Following the launch of the Guidance in 2024, the Secretariat has focused on enabling uptake and implementation at global, regional and country levels. To support this next phase, WHO established the Technical Advisory Group (TAG) on Clinical Research Ecosystem Strengthening, which provides strategic and technical advice to advance implementation efforts.
Global action plan for clinical trials strengthening (GAP-CTS)
In April 2025, the Secretariat published the Global action plan for clinical trials ecosystem strengthening (GAP-CTS), a consensus driven implementation tool anchored in WHO’s 2024 Guidance for best practices for clinical trials and aims to reform and enhance global clinical trial ecosystems.
Led by the WHO Secretariat, this plan sets out nine priority actions to reform how clinical trials are funded, designed, approved, conducted, and reported, making them more efficient, inclusive, and responsive to public health needs.
Why GAP-CTS matters
Many clinical trials today face delays, duplication, and exclusion of key populations. GAP-CTS addresses these challenges by calling for:
- strengthening local leadership and sustainable infrastructure
- streamlining regulatory and ethics reviews
- enhancing community engagement and inclusion
- adopting innovative trial designs and digital technologies
- improving transparency through trial registries
- fostering global collaboration and coordination.
A shared vision for 2030
By 2030, GAP-CTS envisions a globally functional clinical trial ecosystem, with trials embedded in health systems, supported by diverse and trained workforces, and guided by ethical and scientific best practices as outlined in WHO Guidance for best practices for clinical trials.
Join the movement
GAP-CTS is a call to action to all clinical trials stakeholders. Governments, researchers, regulators, funders, patient advocates, ethics committees and communities all have a role to play.
Explore the nine actions and become part of the global effort to strengthen clinical trials and generate better evidence for better health.
Stakeholders are invited to participate in the development and implementation of this transformative plan, ensuring trials produce better-quality evidence to inform policy and improve health outcomes, especially for populations most in need.
Explore the proposed actions and, join this collective effort to reform clinical trial ecosystems globally.
WHO clinical trial capability mapping: strengthening global research ecosystems
The Secretariat is leading a clinical trial capability mapping process. This process involves systematic mapping of national and regional clinical trial capabilities, including legislation, funding, regulatory frameworks, and institutional capacity. By examining data from multiple sources, including the WHO International Clinical Trials Registry Platform (ICTRP), the mapping will identify gaps in clinical trial infrastructure and performance.
The findings will inform coordinated actions to strengthen clinical trial ecosystems globally, supporting Member States and non-state actors to build robust and sustainable ecosystems for evidence generation and effective research oversight. The process is refined in collaboration with research institutes and global experts, ensuring alignment with best practices and evolving needs.
Additional information on this work will be shared in due course.
Developing maturity framework for clinical trial units: enhancing global research capacity
WHO is developing a Maturity Framework for Clinical Trial Units (CTUs), a self-assessment and benchmarking tool designed to strengthen clinical trial capacity and improve research ecosystems globally. Developed in alignment with WHA75.8, the framework will help WHO Member States assess and enhance their clinical research capabilities, guiding them in mapping national stakeholders, identifying gaps, and prioritizing actions for improvement.
The framework will complement other WHO tools and support national health research authorities and institutions in building sustainable, high-quality clinical trial systems. It will foster global and regional collaboration for effective implementation and continuous capacity building.
Empowering global clinical trials through WHO training and resource network
The Secretariat is coordinating the development of a comprehensive online training module and resource network hub to enhance global clinical trial capabilities. This initiative will support the implementation of best practices as outlined in the Guidance for Best practices for clinical trials, developed in response to World Health Assembly resolution WHA75.8.
The training targets key stakeholders, including researchers, regulators, ethics committees, and funders, providing practical tools, peer support, and access to expert resources. By fostering equitable clinical trial capacity, improving health outcomes, and ensuring readiness during emergencies, the training will advance the quality and coordination of clinical research worldwide.
Key milestones so far
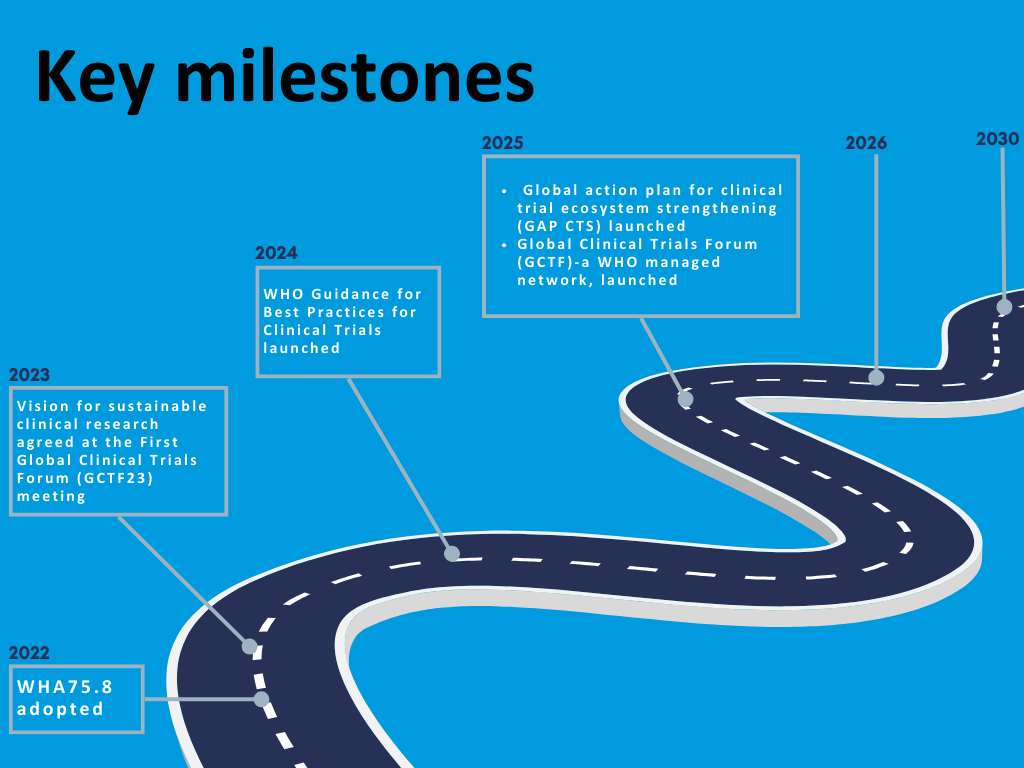
Explore the related sub-pages to discover how the WHA75.8 resolution and WHO's initiatives are shaping the future of clinical trials, addressing global challenges, and fostering collaboration to advance equitable, high-quality health research.
For further information on our work, contact WHA758@who.int
