Mpox Case Investigation Form (CIF) and minimum dataset Case Reporting Form (CRF)
Overview
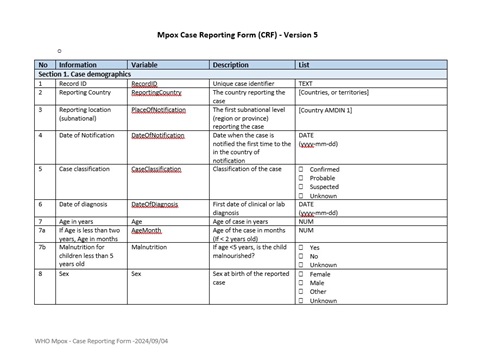
WHO has updated the mpox Case Reporting Form (CRF) and data collection tool to better capture information relevant to the current phase of the multi-country mpox outbreak. The updates include modifications to existing variables, the re-introduction of some variables that were used in the earlier phase of the global outbreak, and the addition of new variables. A detailed list of these changes can be found in the form. Correspondingly, the content of the Case Investigation Form (CIF) has also been revised to reflect these updates.
The CIF is designed as a tool for Member States and researchers to conduct comprehensive epidemiological investigations of suspected, probable, and confirmed mpox cases. It allows for the prospective or retrospective collection of information on mpox cases and their contacts. The full form is intended for in-country use, and the data collected through the CIF does not need to be reported to WHO.
The CRF, on the other hand, serves as a minimum dataset that captures key epidemiological variables on mpox cases. It facilitates reporting to WHO under Article 6 of the mpox standing recommendations and the Public Health Emergency of International Concern (PHEIC) Temporary Recommendations, all aimed at supporting global situational awareness and reporting. To streamline data entry, the data collection tool is available in both MS Word format and MS Excel format, with embedded macro functions (.xlsm format).
Of note, all variables included in the CRF variables are also part of the CIF. Member States are requested to submit the minimum dataset for all cases that meet the definitions of probable or confirmed mpox cases. These submissions should be made through their IHR National Focal Points to their respective WHO Regional IHR Focal Points at least once a month. For countries, regions, and territories with limited laboratory testing capacity, WHO recommends that the CRF also be completed and shared for suspected cases, even if samples are not collected for laboratory testing. This data will be shared in aggregate form in WHO information products.
If you need support please contact emergency-surveillance@who.int
