Sampling for Severe Acute Respiratory Syndrome (SARS) diagnostic tests
Overview
At present, no validated specific diagnostic test exists for detection of SARS coronavirus or antibodies. A summary on use of existing laboratory tests and their characteristics can be found at Severe Acute Respiratory Syndrome (SARS), Laboratory diagnostic tests.
Diagnostic samples should be suitable for viral culture, PCR, antigen detection, immuno-staining and/or serological antibody assays. For
research purposes, to enhance understanding of the SARS disease process, WHO recommends that clinicians collect and store sequential samples from patients with SARS for testing when diagnostic tests become readily available. This is particularly important
for the first case(s) recognized in countries that have not previously reported SARS. Data on clinical and contact history should be correlated with laboratory findings to better understand the shedding pattern of the virus and the period of transmissibility.
Summary of sampling possibilities:
For practical reasons and based on evidence provided by WHO laboratory network, upper respiratory specimens are most suitable for virus detection (isolation and RNA detection). Sampling from multiple sites will increase detection rate.
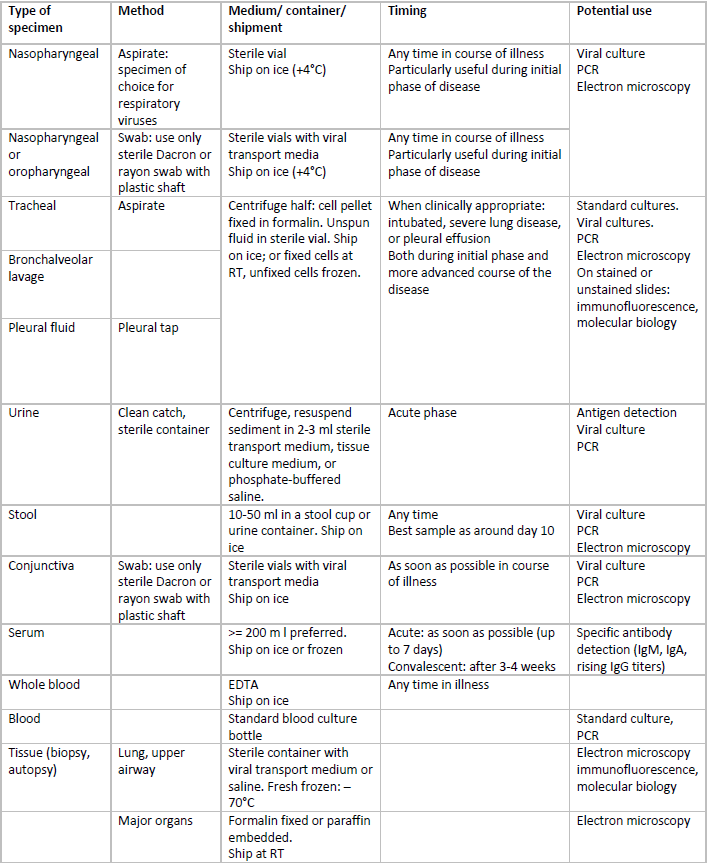
RT: room temperature
Sampling procedures for respiratory tract specimens
a. Nasopharyngeal wash/aspirate
This is the specimen of choice for detection of respiratory viruses. Have the patient sit with head tilted slightly backward. Instill 1 – 1.5 ml of nonbacteriostatic saline into one nostril. Flush a plastic catheter or tubing with 2-3 ml of saline. Insert the tubing into the nostril parallel to the palate. Aspirate nasopharyngeal secretions. Repeat this procedure for the other nostril. Collect specimens in sterile vials.
b. Nasopharyngeal swabs
Insert swab into nostril parallel to the palate and leave in place for a few seconds to absorb secretions. Swab both nostrils.
c. Oropharyngeal swabs
Swab both posterior pharynx and tonsillar areas, avoiding tongue.
d. Lower respiratory tract
See above table
Biosafety: Biosafety precautions for handling, processing and shipment of specimens are detailed in WHO biosafety guidelines for handling of SARS specimens
Personal protection equipment should be worn by the health care worker taking specimens from suspect or probable SARS patients: tight fitting mask (high filtering efficiency: N, R, P 95/99/100, FFP 2/3), goggles, gloves and disposable gown Hospital Infection Control Guidance for Severe Acute Respiratory Syndrome (SARS) .
Tubes and vials containing body specimens should be properly sealed to avoid spillage, clearly labelled with a biohazard label, as well as with identity of sample and date of sampling.
