Technical specifications for selection of essential in vitro diagnostics for SARS-CoV-2
Draft version for public consultation
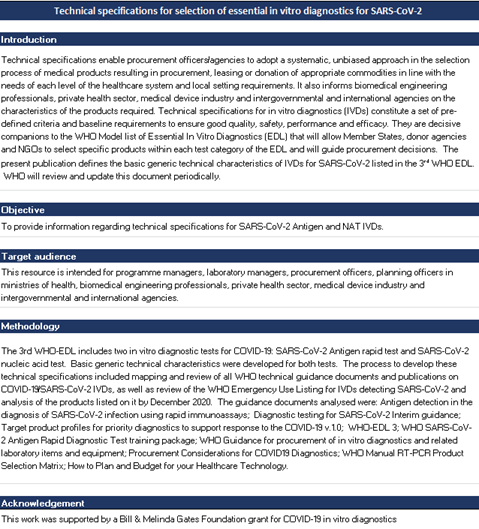
Overview
NOTE: This document is outdated and was intended only for consultation purposes! Please refer to the latest published version: Technical specifications for selection of essential in vitro diagnostics for SARS-CoV-2.
The present file includes 3 technical specification cards for in vitro diagnostics (IVDs) for SARS-CoV-2 listed in the Third WHO model list of in vitro diagnostics (2021):
- Antigen Rapid Diagnostic Test without reader device
- Antigen Rapid Diagnostic Test with reader device
- Nucleic Acid Test.
Each technical specification card is presented in an standard template which is organized as follows: name of the IVD test at the top, and 91 technical specifications organized into 13 fields:
- Name, category and coding
- Intended use
- Performance characteristics
- Technical and operational characteristics
- Instrument physical and technical characteristics
- Infrastructure requirements
- Accessories, consumables, spare parts and other components
- Documentation
- Environmental and safety requirements
- Training, installation and utilisation
- Warranty and maintenance
- Decommissioning
- Quality and registration.
In order to review the technical specification card of your interest go to relevant tab and click on it. The card of the selected item will display in the screen. Scroll down to review the basic generic technical characteristics of this item and please write your comments in the column titled “Comments for review”. Please include your name and affiliation to the reviewed document and add any relevant references.
