
1.4 National TB prevalence surveys
Rationale: why and where are surveys relevant?
To reliably track the burden of tuberculosis (TB) disease in terms of TB incidence and TB mortality from subnational to global levels, the ultimate goal is that all countries can rely on data routinely collected through a) national disease surveillance systems and b) vital registration (VR) systems in which causes of death are coded according to the international classification of diseases (ICD).
Currently, all countries have national systems for notification (i.e. reporting) of people diagnosed with TB and almost all countries report TB case notification data to the World Health Organization (WHO) on an annual basis (Section 2.1). However, in many countries (including most high TB burden countries) the number of notified cases each year is not a good proxy for the actual number of people who develop TB disease, for two reasons. The first is underreporting of people diagnosed with TB, especially in countries with large private sectors or in which people with TB seek care in public facilities that are not linked to the national TB programme and its associated reporting systems. The second is underdiagnosis, especially in countries with geographic or financial barriers to seeking and accessing health care. Many countries (including most high TB burden countries) do not have established national VR systems of high quality and coverage that can be used to reliably monitor the number of deaths and their cause (1).
In countries with a relatively high burden of TB disease that do not yet have national disease notification systems or national VR systems with cause-of-death data that are of sufficiently high quality and coverage, national TB prevalence surveys are the best way to directly measure the burden of TB disease in the population (2–5). In terms of disease burden, WHO currently recommends consideration of surveys according to previously documented epidemiological criteria (2, 3, 5). These criteria, and the countries that currently meet them, are shown in Box 1.4.1.
Box 1.4.1
Criteria for assessment of whether a national TB prevalence survey is relevant
A. Countries that have conducted at least one survey since 2007
Prevalence of bacteriologically confirmed pulmonary TB ≥250 per 100 000 population aged ≥15 years estimated by the most recent survey. This ensures that the sample size will be <70 000 individuals, such that a survey is feasible in terms of cost and logistics.
At least 10 years have elapsed since the most recent survey. This ensures that enough time has passed to allow measurement of a statistically significant change in prevalence between the two surveys.
The 16 countries that currently meet both criteria are Bangladesh, the Democratic People’s Republic of Korea, Ethiopia, Ghana, Indonesia, Kenya, the Lao People’s Democratic Republic, Malawi, Mongolia, Nigeria, Pakistan, the Philippines, Uganda, the United Republic of Tanzania, Zambia and Zimbabwe.
A further 9 countries will meet both criteria during the period 2027—2030: Eswatini, India, Lesotho, Mozambique, Myanmar, Namibia, Nepal, South Africa and Viet Nam.
B. Countries that have never conducted a survey
Estimated TB incidence ≥150 per 100 000 population per year (all forms, all ages). This ensures that the sample size will be <70 000 individuals, such that a survey is feasible in terms of cost and logistics.
No national or sample vital registration (VR) system of high coverage and quality is available. VR systems need to include coding of causes of deaths according to international standards. Without such a system there is no reliable direct measurement of TB disease burden.
Universal health coverage (UHC) service coverage index score is <80 (SDG Indicator 3.8.1) (6). This is an indirect indicator of insufficient access to quality health services, as defined in the WHO TB surveillance checklist of standards and benchmarks (second edition) (7).
The 25 countries that currently meet all three criteria are Afghanistan, Angola, Bhutan, Botswana, Cameroon, the Central African Republic, Chad, the Congo, Côte d’Ivoire, the Democratic Republic of the Congo, Djibouti, Equatorial Guinea, Gabon, Guinea, Guinea-Bissau, Haiti, Kiribati, Liberia, Madagascar, Marshall Islands, Micronesia, Papua New Guinea, Sierra Leone, Somalia and Tuvalu.
What is measured in a survey?
National TB prevalence surveys can provide a reliable measurement of the number of people in the population with bacteriologically confirmed pulmonary TB at a given point in time, and the distribution of these cases by age and sex. In addition, repeat surveys allow assessment of trends, and of the impact of interventions to reduce the burden of disease in the period since the last survey. WHO recommends that surveys focus on people aged 15 years or over (2, 3).
How can survey results be used?
Results can be used to inform national estimates of TB incidence in all age groups, and can thus help to track progress towards the milestones and targets for reductions in TB incidence set in the WHO End TB Strategy (Section 1.1). Previously, survey results were also important for the assessment of progress towards global, regional and national targets for reductions in TB prevalence between 1990 and 2015.
For these reasons, the implementation of national TB prevalence surveys in 22 priority countries (referred to as global focus countries, GFCs) was one of three strategic areas of work defined by the WHO Global Task Force on TB Impact Measurement (the Task Force) for the period 2007–2015 (2, 4). National TB prevalence surveys were retained within the Task Force’s updated strategic areas of work after 2015 (5, 8).
Other benefits of prevalence surveys include:
They provide reliable evidence about the distribution of the burden of TB disease by age and sex; this may be different from the distribution suggested by case notification data.
They allow assessment of how case detection gaps vary by age and sex. This can be done by comparing the ratio of prevalence to notifications by age group and sex. Such evidence can potentially inform the development and implementation of policies or interventions to narrow these gaps.
They provide information about the symptomatic status of people with TB disease in the community, since all survey participants are initially screened using both a chest X-ray and an interview about symptoms. Prevalence survey data have been the key source of data recently used to highlight and discuss the implications of “asymptomatic TB” (see also the featured topic on asymptomatic TB).
Surveys can be used to collect data about the health care seeking behaviour of people with TB disease, and in turn assessment of what improvements to health services may be required to ensure more prompt TB diagnosis and treatment.
Repeat surveys can be used to assess trends in TB disease burden and to evaluate the impact of interventions implemented since the last survey.
If data for people identified to be on TB treatment at the time of the survey are compared with official notification data, the level of underreporting of people diagnosed with TB to the national surveillance system can be assessed. Results can be used to inform the design and implementation of corrective actions, if required.
Survey findings can be used to advocate for action needed to improve TB prevention, diagnosis and treatment, and associated resource mobilization.
Status of progress
National TB prevalence surveys became a strategic area of work for the WHO Global Task Force on TB Impact Measurement in 2007. Between 2007 and August 2024, a total of 36 national TB prevalence surveys in 32 countries were implemented using the screening and diagnostic methods recommended by WHO (Fig. 1.4.1). These 32 countries comprised 17 in Africa and 15 in Asia, and 20 of the 22 GFCs. During this period, five countries implemented repeat surveys: China, Cambodia, Myanmar, the Philippines and Viet Nam. Timor-Leste completed its first survey in December 2023, and Cambodia completed its third survey in May 2024 (Fig. 1.4.2). These numbers represent a major surge in the number of surveys implemented worldwide, following two decades in which only a few surveys were completed, mostly in the WHO Western Pacific Region (4).
Countries that are actively interested in implementing a repeat prevalence survey before 2030 include Ethiopia, Ghana, Malawi, Nigeria, Uganda, the United Republic of Tanzania, Zambia and Zimbabwe in Africa; and Bangladesh, Indonesia, Pakistan and Thailand in Asia. Botswana is interested in implementing a first survey.
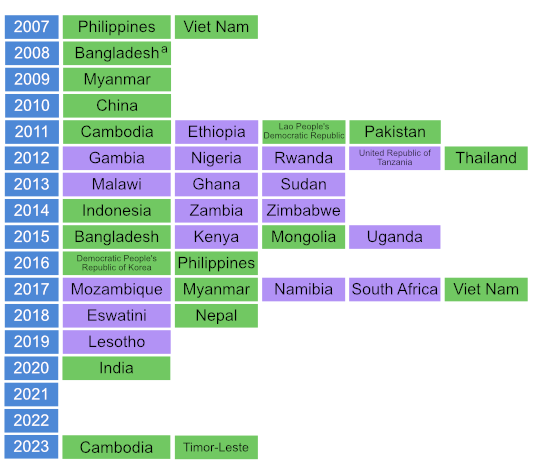
Fig. 1.4.2 Countries in which national population-based surveys of the prevalence of TB disease were completed using WHO-recommended screening and diagnostic methodsa between 2007 and 2024 or where a first or repeat survey is currently being planned or is under considerationb (status in August 2024)
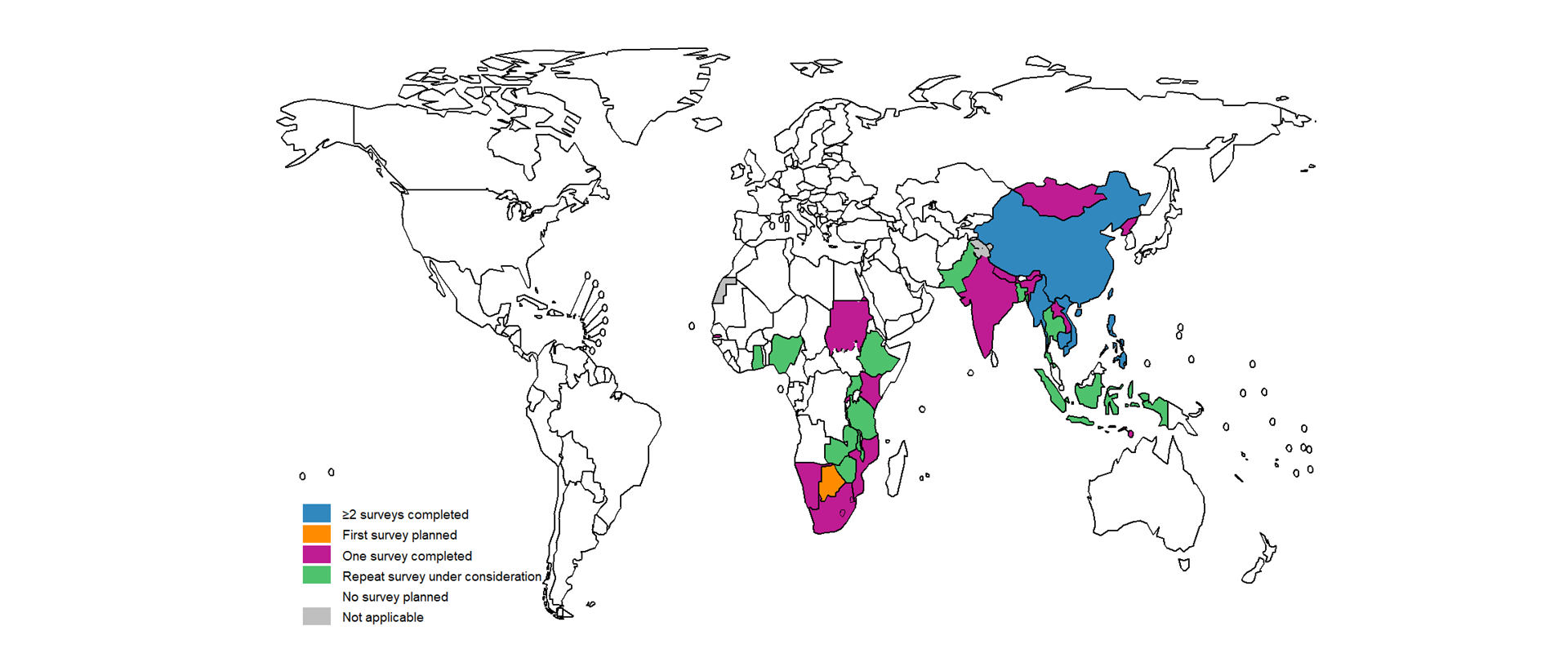
b Repeat survey under consideration: a country has developed a protocol (e.g. Pakistan, Uganda) or expressed strong interest in conducting a repeat TB prevalence survey.
Survey findings and implications
Surveys have shown that the estimated prevalence of bacteriologically confirmed pulmonary TB per 100 000 population aged 15 years or over was high in many countries, but there was also considerable variation (Fig. 1.4.3).
In African countries, prevalence ranged from 119 (95% confidence interval [CI]: 79–160) per 100 000 population in Rwanda (in 2012) to 852 (95% CI: 679–1026) per 100 000 population in South Africa (in 2017). In Asian countries, prevalence ranged from 119 (95% CI: 103–135) per 100 000 population in China (in 2010) to 1159 (95% CI: 1016–1301) per 100 000 population in the Philippines (in 2016).
In most Asian countries and some African countries, prevalence increased with age (Fig. 1.4.4, Fig. 1.4.5).
As transmission declines, more incident cases arise from old
(remote) rather than recent infection. Therefore, a pattern in which
prevalence increases with age suggests that transmission is falling. It
is encouraging that prevalence surveys indicated that transmission is
potentially declining in many Asian countries and in several African
countries (e.g. Ghana, Lesotho, Malawi, Mozambique, Rwanda and the
United Republic of Tanzania). Elsewhere, surveys suggested considerable
community transmission; peaks in many African countries in the age
groups 35–44 or 45–54 years also reflect the impact of the HIV
epidemic.
A striking finding across all surveys was the much higher burden of TB disease in men compared with women (Fig. 1.4.6). The male to female (M:F) ratio of bacteriologically confirmed pulmonary cases in surveys completed in 2007–2021 ranged from 1.2 (in Ethiopia) to 4.5 (in Viet Nam); in most countries it was in the range 2–4. These findings mean that men typically account for about 66–75% of the burden of TB disease in adults.
Ratios of prevalence to notifications (P:N, expressed in
years) suggest marginally higher detection and reporting gaps in Asia
compared with Africa, and lower detection and reporting gaps among women
compared with men (Fig.
1.4.7, Fig.
1.4.8). The combination of a higher disease burden in men and
larger gaps in detection and reporting indicates a need for strategies
to improve access to and use of health services among men
(9).
A large proportion of survey participants with
bacteriologically confirmed pulmonary TB did not report symptoms during
screening and were only tested for TB based on their chest X-ray
results. This proportion was higher in Asian countries compared with
African countries, and varied from 30% in Malawi to 86% in Myanmar
(Fig. 1.4.9). The
classification and natural history of these individuals, their
contribution to overall transmission at the population level, and
implications for case-finding and treatment warrants further exploration
(10–15). For more
information about “asymptomatic TB” please refer to the featured topic on asymptomatic TB.
Data about health care seeking behaviour among people who reported symptoms that met survey screening criteria are available for 27 countries. Of those who had sought care prior to the survey, most initially sought care at public health facilities; this was followed by pharmacies, a mix of sources classified as “other” (e.g. traditional healer) and private health facilities (Fig. 1.4.10).
About half of survey participants who reported symptoms that met survey screening criteria had not sought care for their symptoms (Fig. 1.4.11). Many survey participants regarded their symptoms as not important enough to seek assistance, or faced geographical or financial barriers to accessing care. Addressing barriers to prompt diagnosis (e.g. the costs of accessing care, insufficient availability of rapid diagnostic tests at the places where people initially seek care, insufficient health care worker awareness about TB) is essential for the prompt diagnosis and treatment of people with TB.
For more information
A WHO publication provides full details about the results and lessons learned from the 25 national surveys implemented between 2007 and 2016 (4). This includes additional information about how repeat surveys have been used to assess trends (in Cambodia, China and the Philippines) and to measure the level of underreporting and to take corrective actions (in Indonesia). In addition, regional syntheses of survey results and lessons learned are available in journal articles (16, 17). Country case studies to showcase the results from surveys and how they have been used have also been included in previous editions of the Global TB Report (e.g. Myanmar and Viet Nam in 2019 (18), the Philippines in 2017 (19), Indonesia in 2015 (20), Nigeria in 2014 (21)).
A WHO publication in May 2023 discusses the diagnostic algorithms to be used in future surveys (implemented from 2023 onwards) (22). It provides the basis for recommendations on diagnostic algorithms included in the third edition of WHO guidance on national TB prevalence surveys which will be published later in 2024 (3).
References
Mikkelsen L, Phillips DE, AbouZahr C, Setel PW, de Savigny D, Lozano R et al. A global assessment of civil registration and vital statistics systems: monitoring data quality and progress. Lancet. 2015;386(10001):1395-406. (https://doi.org/10.1016/S0140-6736(15)60171-4).
Tuberculosis prevalence surveys: a handbook (WHO/HTM/TB/2010.17). Geneva: World Health Organization; 2011 (https://iris.who.int/handle/10665/44481).
World Health Organization. Consolidated guidance on tuberculosis data generation and use: module 3: national tuberculosis prevalence surveys. Geneva, Switzerland: World Health Organization; 2024 (in press).
National tuberculosis prevalence surveys, 2007-2016. Geneva: World Health Organization; 2021 (https://iris.who.int/handle/10665/341072).
World Health Organization Global Task Force on TB Impact Measurement. Report of the sixth meeting of the full Task Force; 19-21 April 2016, Glion-sur-Montreux, Switzerland. Geneva: World Health Organization; 2016 (https://cdn.who.int/media/docs/default-source/hq-tuberculosis/global-task-force-on-tb-impact-measurement/meetings/2016-05/tf6_report.pdf?sfvrsn=8a9563a3_3).
Sustainable development report – SDG 3 indicator: universal health coverage (UHC) index of service coverage [website]. 2023 (https://data.who.int/indicators/i/3805B1E/9A706FD).
Consolidated guidelines on tuberculosis. Module 1: Tuberculosis surveillance. Web Annex B. Standards and benchmarks for tuberculosis surveillance and vital registration systems: checklist (2nd edition). Geneva: World Health Organization; 2024 (https://iris.who.int/handle/10665/376483).
Fact sheet on the WHO Global Task Force on TB Impact Measurement (July 2024). Geneva: World Health Organization; 2024 (https://www.who.int/publications/m/item/WHO-UCN-TB-2024.5).
Horton KC, MacPherson P, Houben RM, White RG, Corbett EL. Sex differences in tuberculosis burden and notifications in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med. 2016;13(9):e1002119 (https://doi.org/10.1371/journal.pmed.1002119).
Kendall EA, Shrestha S, Dowdy DW. The Epidemiological Importance of Subclinical Tuberculosis. A Critical Reappraisal. Am J Respir Crit Care Med. 2021 Jan 15;203(2):168-174. (https://doi.org/10.1164/rccm.202006-2394PP).
Richards AS, Sossen B, Emery JC, Horton KC, Heinsohn T, Frascella B et al. Quantifying progression and regression across the spectrum of pulmonary tuberculosis: a data synthesis study. Lancet Glob Health. 2023 May;11(5):e684-e692. doi: 10.1016/S2214-109X(23)00082-7. Epub 2023 Mar 23. (https://doi.org/10.1016/S2214-109X(23)00082-7)
Sossen B, Richards AS, Heinsohn T, Frascella B, Balzarini F, Oradini-Alacreu A et al. The natural history of untreated pulmonary tuberculosis in adults: a systematic review and meta-analysis. Lancet Respir Med. 2023;11:367-79. (https://doi.org/10.1016/S2213-2600(23)00097-8).
Horton KC, Richards AS, Emery JC, Esmail H, Houben R. Reevaluating progression and pathways following Mycobacterium tuberculosis infection within the spectrum of tuberculosis. Proc Natl Acad Sci U S A. 2023;120:e2221186120. (https://doi.org/10.1073/pnas.2221186120).
Stuck L, Klinkenberg E, Abdelgadir Ali N, Ahmed Basheir Abukaraig E, Adusi-Poku Y, Alebachew Wagaw Z et al. Prevalence of subclinical pulmonary tuberculosis in community settings: an individual participant data meta-analysis. The Lancet Infectious Diseases. 2024. (https://doi.org/10.1016/S1473-3099(24)00011-2).
Coussens AK, Zaidi SMA, Allwood BW, Dewan PK, Gray G, Kohli M et al. Classification of early tuberculosis states to guide research for improved care and prevention: an international Delphi consensus exercise. Lancet Respir Med. 2024. (https://doi.org/10.1016/S2213-2600(24)00028-6).
Onozaki I, Law I, Sismanidis C, Zignol M, Glaziou P, Floyd K. National tuberculosis prevalence surveys in Asia, 1990-2012: an overview of results and lessons learned. Trop Med Int Health. 2015 Sep;20(9):1128-1145 (https://doi.org/10.1111/tmi.12534).
Law I, Floyd K, African TB Prevalence Survey Group. National tuberculosis prevalence surveys in Africa, 2008-2016: an overview of results and lessons learned. Trop Med Int Health. 2020 Nov;25(11):1308-1327 (https://doi.org/10.1111/tmi.13485).
World Health Organization. Global Tuberculosis Report. Geneva, Switzerland; 2019 (https://iris.who.int/handle/10665/329368).
World Health Organization. Global Tuberculosis Report Geneva, Switzerland; 2017 (https://iris.who.int/handle/10665/259366).
World Health Organization. Global Tuberculosis Report. Geneva, Switzerland; 2015 (https://iris.who.int/handle/10665/191102).
World Health Organization. Global Tuberculosis Report. Geneva, Switzerland; 2014 (https://iris.who.int/handle/10665/137094).
National tuberculosis prevalence surveys: what diagnostic algorithms should be used in future? Geneva: World Health Organization; 2023 (https://iris.who.int/handle/10665/367909).
