ATC/DDD History
How did ATC/DDD start?
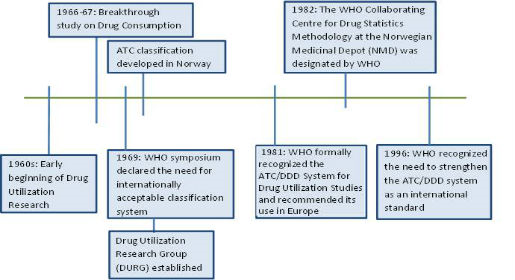
In order to analyze drug use, it is essential to have both a classification system and a unit of measurement. To address the drawbacks of traditional units of measurement, a technical unit of measurement called the Defined Daily Dose (DDD) was developed for use in drug utilization studies.
International interests in the ATC/DDD methodology rapidly expanded, largely through the activity of the DURG. In 1981, the WHO Regional Office for Europe formally recognized the ATC/DDD system for drug utilization studies and recommended its use in Europe. In 1982 the WHO Collaborating Centre for Drug Statistics Methodology was established and assigned the responsibility to coordinate the development and use of the ATC/DDD methodology. In 1996, WHO recommended the global use of the ATC/DDD methodology. Several decades of experience have demonstrated its suitability in drug utilization monitoring and research. The increase in the number of users indicates the usefulness of the system.
1. Engel A, Siderius P. The consumption of drugs. Report on a study, 1966-1967. WHO Regional Office for Europe, Copenhagen 1968 (EURO 3101).
