Situation at a glance
Description of the situation
Since the lifting of the second PHEIC for mpox on 5 September 2025, and as of 24 November 2025, 43 new confirmed cases of clade Ib MPXV have been reported across six WHO regions outside areas where sustained community transmission of this virus strain has been occurring. In four of these regions (Region of the Americas, South-East Asia Region, European Region and the Western Pacific Region), 24 cases had reported no recent international travel, suggesting local transmission. Based on this, Italy, Malaysia, the Netherlands, Portugal, Spain, and the United States of America are now considered to be experiencing community transmission of clade Ib MPXV. In addition, travel-related cases continue to be reported in many countries.
Among the 43 cases, half (22) were documented among men who have sex with men, while other cases were linked to travel to countries with known community transmission of clade Ib, or secondary to travel-related cases (household contacts and/or sexual partners).
This report provides an overview of these recent cases of mpox confirmed to be due to clade Ib MPXV, by WHO region and country, summarizing key available epidemiological information, followed by WHO’s rapid risk assessment and public health advice.
Summary of reported mpox due to clade Ib MPXV in WHO Regions and countries from 5 September to 24 November 2025
WHO African Region
Since the lifting of the PHEIC on 5 September 2025 and as of 24 November 2025, one country, Namibia, has reported clade Ib MPXV cases for the first time. Community transmission persists in Burundi, the Democratic Republic of the Congo, Kenya, Malawi, Mozambique, Republic of Congo, Rwanda, South Africa, the United Republic of Tanzania, Uganda, and Zambia.
Namibia
Namibia notified WHO of one probable and two confirmed cases of mpox due to clade Ib MPXV. The index (probable) case linked to travel within the African Region and the two confirmed cases were his household contacts. No further cases have been reported following detection of this cluster.
These are the first cases of mpox reported in the country.
WHO Region of the Americas
Two countries in the WHO Americas Region have reported a total of four confirmed cases of mpox due to clade Ib MPXV. One case detected in Canada had recently travelled, while three cases in the United States of America had no recent travel history or known epidemiological links to travellers.
Canada
Canada notified WHO of one confirmed mpox case due to clade Ib MPXV in an adult male with recent travel outside of the country and reporting no sexual partners after returning to Canada. The case received counselling on preventing further transmission.
United States of America
The United States of America reported three unrelated cases of mpox due to clade Ib MPXV in Long Beach (one case) and Los Angeles (two cases) counties, California. All three occurred among men who have sex with men, none of whom had a history of recent international travel or known exposure to mpox cases. None of the individuals had a previous MPXV infection or prior orthopoxvirus vaccination, and one case was immunocompromised. All three individuals were hospitalized, received standard medical care, and have fully recovered. Prior to the lifting of the PHEIC, the United States of America had reported six cases of mpox due to clade Ib MPXV, all linked to travel.
Public health authorities conducted contact tracing among household, healthcare-facility and social contacts. No additional cases of mpox due to clade Ib MPXV have been detected to date. Public health investigations suggest ongoing community transmission of clade Ib MPXV among men who have sex with men and their social networks in southern California. Viral genomic sequencing data indicate that the three California cases may be linked to a previously reported case in the country in August 2025.
WHO South-East Asia Region
From 5 September to 24 November 2025, five cases of mpox due to clade Ib MPXV have been reported in the WHO South-East Asia Region, all in Thailand. All cases had a recent history of international travel and three self-identified as men who have sex with men.
Thailand
Thailand notified WHO of five new cases of mpox cases due to clade Ib MPXV. The cases included four males, three of whom self-identified as men who have sex with men, and one female. Travel histories indicate associations with recent travel to the United Arab Emirates, Oman, and the Russian Federation, where exposure to infection is likely to have occurred. Prior to 5 September, Thailand had reported five cases of mpox due to clade Ib MPXV, all of which were associated with international travel.
WHO Eastern Mediterranean Region
Three countries in the WHO Eastern Mediterranean Region, Egypt, Lebanon and Qatar have reported six cases of mpox. Although the clade was not documented in Egypt and Lebanon, two cases attributed to clade Ib MPXV were reported in Qatar.
Qatar
Qatar notified WHO of two cases of mpox due to clade Ib MPXV. One adult male and one adult female, linked to travel within the Eastern Mediterranean Region. Prior to this period, Qatar had reported three cases of mpox due to clade Ib MPXV, all of which were associated with international travel.
WHO European Region
Countries in the WHO European Region have reported a total of 27 mpox cases due to clade Ib MPXV. Of these, 18 cases were classified as autochthonous, with no relevant history of recent international travel, suggesting undetected community transmission (Italy, the Netherlands, Portugal, and Spain). Two cases (reported from Belgium and the United Kingdom) were related to travel within Europe and five cases to travel outside of Europe (East Africa, Uganda, United Arab Emirates), either to or from countries experiencing community transmission of clade Ib MPXV but also to or from countries where no community transmission has been reported, including Angola, the United Arab Emirates, and Viet Nam. Furthermore, at least 15 of the 27 cases, and 14 of the 18 locally acquired cases occurred among individuals who self-identified as men who have sex with men.
Belgium
Belgium reported to WHO one case of mpox due to clade Ib MPXV with recent travel to the Netherlands. This individual reported having had multiple sexual contacts with other men while in the Netherlands. Prior to 5 September, Belgium had reported six mpox cases caused by clade Ib MPXV, all linked to travel.
France
France notified WHO of one case of mpox due to clade Ib MPXV in an adult male traveller who had returned from East Africa. Prior to this period, France had reported three cases of mpox due to clade Ib MPXV, all linked to travel.
Germany
Germany notified WHO of three cases of mpox due to clade Ib MPXV. All three cases had a recent history of international travel: one, an adult male who had travelled to Angola, another an adult female who had travelled to Uganda, and the third, an adult male who had travelled to Viet Nam. Uganda has community transmission of clade Ib and Viet Nam has not previously reported cases of this subclade. Prior to 5 September, Germany had reported 12 mpox cases due to clade Ib MPXV, most of which were linked to travel.
Greece
Greece notified WHO of its first case of mpox due to clade Ib MPXV, in an adult male with a recent history of travel to the United Arab Emirates before arriving in Greece.
Ireland
Ireland reported two cases linked to a small cluster which was reported before 5 September 2025. The index case had history of recent travel outside Europe. The first locally acquired case was a child (<5 years) who contracted clade Ib MPXV through household transmission, and the second a healthcare worker who had cared for one of the earlier cluster cases. No additional cases have been identified following this localized clade Ib cluster. Prior to this period, Ireland had reported one confirmed case of mpox due to clade Ib MPXV, which was linked to a traveller who was a probable case and part of that same cluster.
Italy
Italy notified WHO of two cases of mpox due to clade Ib MPXV in adult males who had no history of recent international travel or known contact with mpox cases. There was no epidemiological link between these cases. Epidemiological investigations and contact tracing were conducted, and no additional cases were identified.
The identification of these cases of mpox due to clade Ib MPXV in Italy, without any links to travel, suggests local community transmission of clade Ib MPXV in the country. Prior to this period, Italy had reported one case of mpox due to clade Ib MPXV, linked to travel the United Republic of Tanzania.
The Netherlands
The Netherlands has reported nine cases of mpox due to clade Ib MPXV, all without relevant history of recent travel. These are the first such cases of mpox reported in the country. Eight cases were reported among individuals who identify as men who have sex with men. Of these, six individuals reported visiting the same highly frequented sex-on-premises venue. These cases suggest local community transmission of clade Ib MPXV in the country. Prior to the 5 September, the Netherlands had not reported any cases of mpox due to clade Ib MPXV.
Portugal
Portugal notified WHO of its first confirmed case of mpox case due to clade Ib MPXV. The case is an adult male with no history of recent international travel, with an inconsistent exposure context, and no known link to a case. There were no identified contacts, and the case was provided with guidance on home isolation, suspension of all sexual contact, and adherence to hygiene measures until full lesion resolution. Subsequently, the case ceased communication with health authorities.
In Portugal, outbreak prevention and control measures are still ongoing at national and subnational levels. Reinforcement of clinical, laboratory and epidemiological detection, as well as engagement with civil society and the most at-risk communities promoting vaccination, has been in place. No further cases of mpox due to clade Ib have been detected in Portugal.
The identification of this case of mpox due to clade Ib MPXV in Portugal, without any link to travel, and although no other cases have been detected, suggests local community transmission of clade Ib MPXV in the country.
Spain
Spain reported six cases of mpox due to clade Ib MPXV in individuals with no recent history of international travel or known contact with mpox cases. Two additional I MPXV cases were reported without further subclade information. These are the first cases of mpox due to clade Ib MPXV reported in the country. All cases were among individuals who identify as men who have sex with men. The identification of cases of mpox due to clade Ib MPXV in Spain, without any link to travel, suggests local community transmission of clade Ib MPXV in the country.
The United Kingdom of Great Britain and Northern Ireland
The United Kingdom notified WHO of one case of mpox due to clade Ib MPXV with travel history within Europe. This patient was a man who reported having sex with other men. Prior to this case, the United Kingdom had reported 18 clade Ib cases, most of whom reported direct or indirect links to travel to countries where clade Ib MPXV is circulating.
WHO Western Pacific Region
From 5 September to 24 November 2025, three cases of mpox due to clade Ib MPXV have been reported in the WHO Western Pacific Region: one each in Australia, Japan, and Malaysia.
Australia
Australia notified WHO of one case of mpox due to clade Ib MPXV in an adult male who reported recent travel to China and to the Philippines, where he was most likely infected. The Philippines have not reported any cases of mpox due to clade Ib MPXV. Prior to 5 September, three cases of mpox due to clade Ib MPXV, all linked to travel, had been reported in Australia.
Japan
Japan notified WHO of its first case of mpox due to clade Ib MPXV in adult female who reported recent travel to Africa, where she was most likely exposed to the virus.
Malaysia
Malaysia notified WHO of its first case of mpox due to clade Ib MPXV in an adult male, who self-identified as a man who has sex with men. He had no history of recent international travel nor any link to a known case. The person reported sexual contact with at least one individual during the three weeks prior to symptom onset. Contact tracing identified 15 household and healthcare contacts who underwent monitoring and no additional cases followed. The identification of this case of mpox due to clade Ib MPXV in Malaysia, without any link to travel, suggests local transmission of clade Ib MPXV in the country.
Table 1. Summary of mpox due to clade Ib MPXV, by country, 5 September to 24 November 2025.
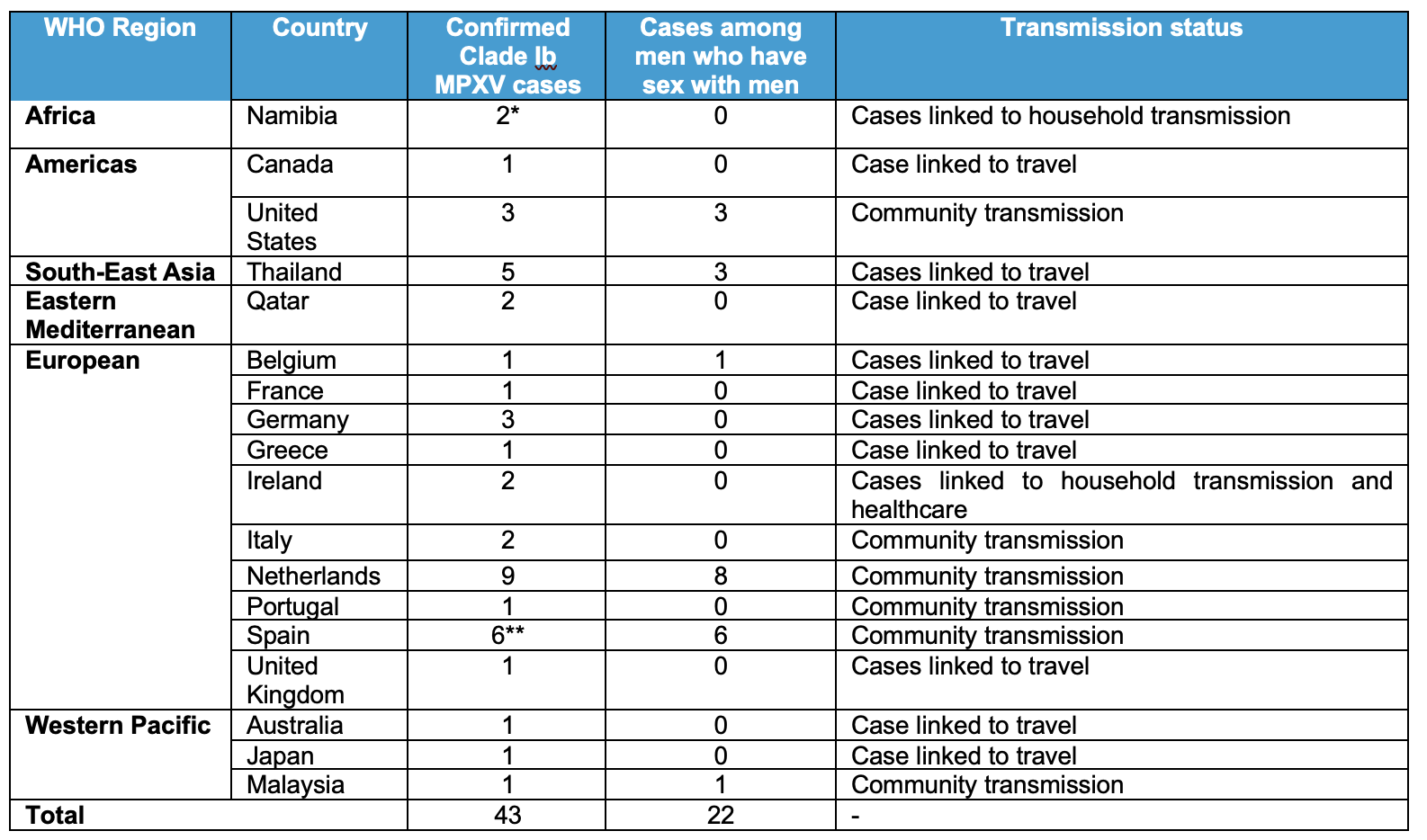
*One additional case is not yet confirmed; therefore, it is not included in the table.
**Two additional cases of mpox due to clade I MPXV among MSM, with no recent travel, did not have subclade information available
Epidemiology
Mpox is an infectious disease caused by the MPXV, divided into two clades, clade I (including subclades Ia and Ib), and clade II (including subclades IIa and IIb. Historically associated with zoonotic transmission in tropical rainforest regions of East, Central and West Africa, mpox has in recent years predominantly spread through human-to-human transmission and has rapidly emerged across all WHO regions. Subclades Ia and Ib have been following the emergence of clade Ib in South Kivu province of the Democratic Republic of the Congo in 2023. Clade Ia is currently considered to encompass all other strains of Clade I that are not Ib. Clade IIb continues to circulate in all WHO regions since 2022.
The virus is primarily transmitted through close physical contact with a person who has mpox, through sexual contact or other forms of direct skin-to-skin contact, for example from parent to child. Other documented routes include indirect contact with contaminated materials, occasionally non-physical contact such as close-range inhalation of infectious respiratory particles, and vertical transmission from mother-to-child during pregnancy or childbirth. In historically endemic areas transmission can also occur from animals to humans through contact with live animals or consumption of contaminated bushmeat. Emerging evidence indicates that exposure to MPXV can result from subclinical infection in another person and silent shedding of the virus, particularly in genital and anal secretions, which can facilitate further transmission during sexual contact.
Mpox causes signs and symptoms which usually begin within 3-7 days of exposure and can start as soon as one or rarely up to 21 days later. Symptoms typically last for two to four weeks but may last longer in someone with a weakened immune system, for example as a result of advanced untreated HIV infection. Fever, muscle aches and sore throat may appear first, followed by an evolving skin rash and/or mucosal lesions, or appearance of such lesions may precede systemic symptoms. Lymphadenopathy (swollen lymph nodes) is also a typical feature of mpox, present in most cases. Transmission through sexual contact has been observed to lead to the appearance sometimes of only genital lesions. Children, pregnant women and people with weak immune systems, most commonly due to advanced HIV infection, are at risk of developing complications and dying of mpox.
Laboratory testing is necessary to confirm mpox, particularly for the first cases in an outbreak or new geographic area. The primary diagnostic test for MPXV infection is polymerase chain reaction (PCR). The best diagnostic specimens are taken directly from lesion material – on skin or mucosae, such as lesion fluid or crusts – collected by vigorous swabbing. In the absence of skin or mucosal lesions, testing can be done on oropharyngeal, anal or rectal swabs. However, while a positive result of oropharyngeal, anal or rectal sample confirms mpox, a negative result is not enough to rule out MPXV infection. Testing of blood is not recommended as any viremia is usually brief and individuals can test negative just a few days after infection. Serology does not distinguish between different orthopoxviruses and is therefore restricted to reference laboratories where antibody detection methods may be applied for retrospective case classification or in special studies.
Treatment is based primarily on managing clinical symptoms, ensuring skin care, eye care, reducing pain, and preventing and managing secondary bacterial infections and other complications. Where available through clinical studies or emergency access protocols, specific antiviral medications may also be used in the treatment of mpox, particularly for severe cases or individuals at higher risk of complications.
Vaccines for use to prevent mpox are available to all countries. WHO recommends use of MVA-BN (non-replicating) or LC16m8 (minimally replicating) vaccine as indicated, or ACAM2000 (replicating) vaccine based on an individual risk-benefit assessment when the others are not available. In the context of an outbreak, vaccination is recommended by WHO for individuals at high risk of exposure to mpox, such as sex workers; gay, bisexual or other men who have sex with men; or other individuals with multiple sexual partners; health workers and frontline workers, contacts of known mpox cases, and other affected groups in a geographically defined area or community (based on local epidemiology).
Public health response
WHO maintains global mpox surveillance and continues to provide response guidance for all countries and support to access diagnostics and vaccines through multi-partner coordination. WHO and partners have established the International Coordinating Group for mpox vaccine provision (ICG) to further accelerate timely outbreak response and ensure sustainable support for the future. Furthermore, WHO continues to evaluate available rapid diagnostic tests for field use.
Public health response measures taken in the affected WHO Regions include:
WHO African Region
- A continental mpox response is ongoing in the region, including all main response pillars.
- Surveillance activities continue, and most countries have mpox diagnostic capacity in place
- Sixteen countries have received mpox vaccines and are vaccinating people at risk to contain the outbreaks.
WHO Region of the Americas
- WHO supports Member States with surveillance, preparedness, and outbreak response in activities for mpox in affected countries.
- WHO provides information through situation reports and the mpox dashboard.
- Vaccines remain available to countries through the PAHO Revolving Fund.
- The response is focused on communication and engagement of at-risk communities, timely detection of cases and treatment of patients, laboratory confirmation, surveillance, and containment of transmission chains, securing access to critical health supplies and protection of health workers.
WHO South-East Asia Region
- WHO provides weekly briefings and technical guidance to Member States to enhance preparedness, including laboratory diagnostics and access to medical countermeasures (MCM).
- Access to MCM is facilitated through allocation and supply chain coordination for diagnostics and support to regulatory preparedness through a regional regulatory network.
- WHO provides guidance on clinical management and infection prevention and control to strengthen capacity for safe and scalable care.
- A collaborative surveillance mechanism for early detection and reporting includes coordination between HIV/STI and emergency programmes. Support includes genomic sequencing, reagent supply, training, and bioinformatics to improve regional capacity.
- Community protection and communication strategies are being scaled up, with targeted outreach to high-risk groups, social listening, rumour tracking, and regional adaptation of messaging, alongside efforts to improve genomic sequencing capacity and regulatory preparedness for diagnostics.
WHO Eastern Mediterranean Region
- WHO continues to support coordination, reporting, and information sharing between countries through the IHR mechanism.
- WHO continues advocating with national stakeholders to integrate mpox prevention and care in routine STI services.
- WHO supports countries to access mpox diagnostics services through facilitating procurement and distribution of kits.
- WHO works closely with national health authorities to strengthen surveillance and ensure inclusion of mpox in lists of diseases for routine surveillance.
WHO European Region
- WHO and the European Centre for Disease Prevention and Control (ECDC) have asked all Member States to report mpox due to clade I MPXV through official International Health Regulations (IHR) and/or surveillance mechanisms.
- WHO has conducted modelling to better understand drivers of mpox transmission in the region.
- ECDC published a threat assessment brief following the detection of local transmission of clade Ib MPXV in the EU/EEA.
- A Risk Communication, Community Engagement, and Infodemic Management package for health workers and travel advice has been shared with Member States through IHR focal points.
- Questions and answers documents on mpox have been updated.
- WHO and ECDC are planning a community briefing on the situation to understand community perceptions.
WHO Western Pacific Region
- Following notification of a locally acquired case due to clade Ib in the Region, and exportation of clade Ib from the Region, WHO has been working with national authorities on epidemiological investigation, contact follow-up up and assessment of potential transmission settings and continues to monitor for additional cases and offer cross-border support.
- WHO continues to support countries with surveillance, preparedness, and investigation of suspected mpox events, including rapid risk assessments, technical advice on case management and IPC, and verification of event information through IHR channels.
- WHO provides targeted support to strengthen diagnostic capacity, including guidance on clinical sampling, access to PCR testing for clade I and clade II MPXV, and coordination with national laboratories to increase genomic sequencing capability for clade and subclade identification.
- WHO is working to integrate mpox preparedness within services for HIV and sexually transmitted infections, promoting early HIV testing and prompt antiretroviral therapy (ART) initiation for any person with mpox, in line with WHO guidance for clinical care and infection prevention and control.
- WHO supports clinical readiness through regional webinars, technical exchanges, and Communities of Practice, enabling countries to access updated clinical management guidance, peer support, and context-specific tools for managing suspected and confirmed cases.
- Risk communication and community engagement activities focus on social listening, information, education and communication (IEC) material production and close collaboration with health workers and key population networks, including men who have sex with men, sex workers, and community-based organizations, to strengthen awareness of symptoms, promote early care seeking, and reduce stigma associated with mpox.
- WHO continues to assist countries in reviewing and strengthening national preparedness measures, including support for intra-action reviews and integration of mpox within all-hazards emergency preparedness and response frameworks.
WHO risk assessment
In light of the epidemiological developments presented above and confirmation of community transmission of clade Ib MPXV in all WHO regions, WHO assesses the public health risk posed by clade Ib MPXV as moderate for men who have sex with men with new and/or multiple partners, and the risk to the general population as low.
The rationale for this assessment is outlined below.
When timely and good quality care is available, mpox generally causes a mild to moderate disease characterized by systemic symptoms and localized skin and/or mucosal lesions. However, secondary bacterial infections and other complications can lead to severe illness and death. In most settings, the proportion of cases where death occurs (case fatality ratio) is below 1%. Risk factors for severe disease and death include immunosuppression from any cause, such as uncontrolled HIV, younger age (0-4 years) and pregnancy. In recent years, people living with untreated or uncontrolled HIV have experienced the highest burden of mpox-related mortality. In African countries, deaths have also occurred among young children, pregnant women and their unborn or newborn infants, and individuals with other immunocompromising conditions.
WHO has twice declared a PHEIC for mpox in recent years. The first, in 2022, was linked to a multi-country outbreak of clade IIb MPXV spreading through sexual networks across all regions of the world, primarily among men who have sex with men who have more than one partner. Transmission beyond this group during the global outbreak remained very limited, and was brought under control in the second half of 2023, with low-level sporadic transmission persisting in the same population, likely due to mild, undetected or subclinical transmission through sexual contact.
The second PHEIC for mpox was declared in 2024 due to the rising number of mpox cases reported in Africa and the spread of the newly identified clade Ib MPXV across several African countries, most of which were affected by mpox for the first time and noted both sexual and non-sexual contact transmission. These outbreaks have led to sustained community transmission of this strain in several countries primarily in Central and East Africa. In many settings, transmission was initially driven by heterosexual contact among people with multiple casual sexual partners in linked sexual networks which included but were not limited to sex workers, followed by secondary spread within households. In most of these settings, virus circulation persists through both recognized and cryptic (undetected or unreported) transmission.
The locally acquired cases of mpox due to clade Ib MPXV described above in individuals in multiple countries and WHO regions suggest that undetected transmission of this subclade is occurring independently in these settings. This transmission is likely accelerated due to some infections with no or minimal symptoms (asymptomatic or paucisymptomatic cases), leading to further onward transmission, predominantly through sexual contact. This hypothesis is supported by the rising proportion of clade Ib MPXV cases among men who have sex with men. It is likely that clade Ib MPXV will continue to spread, and that community transmission will become established in more countries, primarily mediated by sexual contact in extended sexual networks. Men who have sex with men, particularly those with a high number of casual sexual contacts, remain at increased risk of mpox, including clade Ib MPXV infection. Currently, immunity to mpox in this population is a result of vaccination efforts since 2022 in some countries and immunity conferred by exposure to mpox during the clade IIb MPXV outbreak. However, many countries were not able to offer mpox vaccination, access was constrained in some countries where vaccine was available, and many individuals received an incomplete course of vaccination. In addition, since the peak of vaccination activities in late 2022, new cohorts of young individuals have entered the sexually active population. These individuals are more likely to be immunologically naïve having neither had previous infection nor been reached by vaccination activities. Furthermore, available data on vaccine effectiveness, uncertainty around the duration of protection against mpox following vaccination or prior infection, uncertainty regarding cross-clade protection of prior immunity during outbreaks with ongoing virus strain evolution, together with emerging data on waning humoral immunity over time, all limit confidence that those vaccinated or infected in 2022 and 2023 continue to retain protective immunity. Furthermore, of all cases reported by the countries noted here from 5 September to 24 November, only half were among men who have sex with men, suggesting continuing risk in other groups.
All cases reported here are clinically stable and in isolation or recovered from the disease. The known contacts for most of them were followed up for 21 days to ensure early recognition of symptoms and diagnosis. The clinical risk for these cases and their contacts is low, notably if they have been vaccinated and are not immunocompromised.
Data from the global outbreak related to clade IIb, and the multiple importations of clade Ib MPXV in the last year, suggests that transmission of mpox outside sexual networks has been relatively limited in most high-income settings. In this context, the risk of community transmission of mpox across different population groups is still considered to be low. However, given the large outbreaks and extensive community transmission of clade Ib MPXV in Africa affecting different populations, including children, together with associated outbreaks in many countries, it remains critical to maintain vigilance in all regions, and most notably for population groups at higher risk of sexual transmission.
While affected countries have developed the capacity to detect and respond effectively to mpox outbreaks, in-depth epidemiological investigation and contact tracing remain challenging. Individuals with mpox are often reluctant to disclose their exposure history or current sexual contacts, which hinders full mapping of transmission chains and raises the likelihood of undetected onward spread. Prompt isolation of cases, identification and monitoring of reported contacts, and timely administration of post‑exposure vaccination to at-risk contacts, ideally within four days of exposure, all help to reduce the immediate risk of secondary cases. However, given that sexual transmission of clade Ib is now occurring in many countries, the risk is high that clade Ib MPXV will continue to spread in newly affected countries and to other countries around the world.
In light of these recent developments, WHO assesses the public health risk posed by clade Ib MPXV for men who have sex with men with new and/or multiple partners as moderate and to the general population as low. The risk for men is justified by the higher risk of exposure in this population, and the prevalence of advanced HIV infection in this group in many contexts compared to the general population. The higher risk is mitigated by residual protective benefit of previous natural infection and/or prophylactic vaccination in this group in some areas. The extent to which immunity in the group could indirectly benefit younger individuals or those not previously vaccinated or exposed is not known and will continue to diminish over time if access to vaccines is not sustained.
All mpox outbreaks, including individual locally acquired cases, should be assessed in their local context to better understand the epidemiology, transmission patterns, risk factors for severe disease, viral reservoir and evolution, and relevance of strategic approaches and countermeasures for prevention and control. Regardless of geographic area, epidemiological context, gender identity or sexual behaviour, an individual’s risk largely depends on factors such as exposure risk and immune status.
WHO advice
Community transmission of clade Ib MPXV is occurring in many countries within and beyond Africa. WHO strongly advises that countries continue to follow the Standing Recommendations issued in 2023 and extended through 20 August 2026, particularly concerning the epidemiological surveillance of mpox and the strengthening of laboratory diagnostic capacities in line with WHO guidance, together with all other elements of response including risk communication and community engagement and ensuring access to mpox vaccine for people at risk. Countries must have, or arrange access to, diagnostic capacities to detect both MPXV clades and subclades. Public health authorities are strongly encouraged to ensure access to genomic sequencing capacity for virus clade identification for new cases and clusters of cases as part of comprehensive prevention and response measures.
The WHO Strategic framework for enhancing prevention and control of mpox (2024–2027) outlines a road map to prevent and control outbreaks characterized by human-to-human transmission in every context, advance mpox research and access to countermeasures, and minimize zoonotic transmission where relevant in some African countries.
Countries and communities are strongly encouraged to enhance preparedness, foster community ownership, widen access to vaccination for people at risk, and ensure cross-border coordination, especially in regions with mobile and vulnerable populations. In terms of risk communication, community engagement and infodemic management (RCCE-IM), countries are encouraged to:
- continue to engage closely with communities who may be at risk, such as men who have sex with men, sex workers and other groups at risk, to promote uptake of protective measures;
- expand outreach to include diaspora populations from and travellers to countries where mpox is currently circulating, including public health advice at points of entry;
- ensure that all communication and interventions are delivered in a stigma-free, respectful and inclusive manner, avoiding messaging that reinforces negative stereotypes or discrimination.
Mpox vaccines formulated with vaccinia virus provide protection against mpox. WHO recommends vaccination against mpox in the context of an outbreak for people most at risk of exposure to mpox and for preventive use for laboratory personnel working with orthopoxviruses, in line with recommendations of the WHO Strategic Advisory Group of Experts on Immunization (SAGE) and the WHO position paper on mpox vaccines. Two vaccines currently in use for mpox are recommended. MVA-BN (non-replicating vaccine) has been prequalified by WHO and broadly used in outbreak response and LC16m8 (minimally replicating vaccine) has received a WHO emergency use listing (EUL) and is used in Japan and the DRC and has also been used in Colombia. LC16 is contraindicated for use in pregnancy, immunocompromised individuals, and those suffering from a proliferative skin condition. WHO recommends a vaccination for people at risk or where relevant in geographic areas at risk to interrupt transmission. National authorities are encouraged, as a temporary outbreak response measure, to administer MVA-BN vaccine via intradermal injection at one fifth of the dose normally administered subcutaneously (also known as fractional dosing) to protect individuals at risk of exposure and facilitate reaching four to five times as many people. Intradermal vaccination with MVA-BN has been shown to be effective and safe.
Anyone with a clinical or laboratory-confirmed diagnosis of mpox should follow the instructions of local health authorities, including isolation in a health facility or at home for the duration of the infectious period. Persons with mpox should avoid travel, including local and international travel, unless the reason for travel is to seek medical care, until they do not present any mpox symptoms and the scabs have fallen off and a fresh layer of skin has formed underneath. Contacts of a confirmed case are asked to limit their movements (and to abstain from sexual relations) for 21 days, the maximum incubation and monitoring period for the appearance of possible symptoms.
WHO strongly recommends implementation of optimized clinical care for patients with mpox, to reduce the risk of medical complications and long-term sequelae and improve health outcomes. Mpox disproportionally affects people living with HIV, with a higher risk of severe disease, hospitalization or death in people with advanced HIV disease. WHO strongly recommends early HIV testing for all patients with suspected or confirmed mpox and rapid initiation of antiretroviral therapy (ART) in people living with untreated HIV who are diagnosed with mpox.
Health authorities at all levels should provide travellers with information to protect themselves and others before, during and after travel to mpox-affected countries or attending events or gatherings where mpox may present a risk. WHO does not recommend any restriction on travel to or trade with the countries named in this report.
For additional information on WHO public health advice to reduce the risk of mpox please see the resources listed below in the further information section.
Further information
- World Health Organization. Mpox: fact sheet. 2024 Aug 26. Available from: http://www.who.int/news-room/fact-sheets/detail/monkeypox
- World Health Organization. Global mpox trends. Available from: https://worldhealthorg.shinyapps.io/mpx_global/
- World Health Organization. Multi-country outbreak of mpox: external situation report no. 59. 2025 Oct 30 Available from: https://www.who.int/publications/m/item/multi-country-outbreak-of-mpox--external-situation-report--59---30-october-2025
- World Health Organization. Fifth meeting of the International Health Regulations (2005) Emergency Committee regarding the upsurge of mpox 2024. 2025 Oct 30. Available from: https://www.who.int/news/item/30-10-2025-fifth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-upsurge-of-mpox-2024
- World Health Organization. Standing recommendations for mpox issued by the Director-General of the World Health Organization in accordance with the International Health Regulations (2005). 2023 Aug 21. Available from: https://www.who.int/publications/m/item/standing-recommendations-for-mpox-issued-by-the-director-general-of-the-world-health-organization-(who)-in-accordance-with-the-international-health-regulations-(2005)-(ihr)
- World Health Organization. Extension of standing recommendations for mpox by the Director-General of WHO. Available from: https://www.who.int/publications/m/item/extension-of-standing-recommendations-for-mpox-by-the-director-general-of-who
- World Health Organization. Clinical management and infection prevention and control for mpox: living guideline. 2025 May. Available from: https://www.who.int/publications/i/item/B09434
- World Health Organization. WHO recommends rapid treatment initiation for people living with HIV and mpox. 2025 Jul 16. Available from: https://www.who.int/news/item/16-07-2025-who-recommends-rapid-treatment-initiation-for-people-living-with-hiv-and-mpox
- World Health Organization. WHO mpox multi-country rapid risk assessment, version 5. 2025 Oct 13. Available from: https://www.who.int/publications/m/item/who-rapid-risk-assessment---mpox--global-v.5
- World Health Organization. Strategic framework for enhancing prevention and control of mpox (2024–2027). May 2024. Available from: https://www.who.int/publications/i/item/9789240092907
- World Health Organization. Guidance on use of Smallpox and mpox vaccines, including WHO Position paper on mpox vaccines and other resources to support countries https://www.who.int/teams/immunization-vaccines-and-biologicals/diseases/smallpox-and-mpox
- World Health Organization. Frequently Asked Questions (FAQ) on use of fractional dosing with intradermal administration of mpox MVA-BN vaccine in the context of vaccine supply-constrained outbreak response. 19 June 2025. https://www.who.int/publications/m/item/frequently-asked-questions-(faq)-on-use-of-fractional-dosing-with-intradermal-administration-of-mpox-mva-bn-vaccine-in-the-context-of-vaccine-supply-constrained-outbreak-response
- World Health Organization. LC16m8 (live-attenuated freeze-dried vaccinia) smallpox and mpox vaccine. Interim guidance. 2025 April 22. Available from: https://iris.who.int/server/api/core/bitstreams/9b10eb01-fbfd-4f9f-81b7-9c29ddbcc560/content
- World Health Organization. Prequalification of Smallpox and Mpox vaccine (Live Modified Vaccinia Virus Ankara), 2024 September 13. Available from: https://extranet.who.int/prequal/vaccines/p/imvanexr
- World Health Organization. Emergency use listing of LC16m8, 2024 November 19. Available from https://extranet.who.int/prequal/vaccines/lc16-kmb
Public Health Advice Resources
- World Health Organization. Public health advice on protecting yourself and others from mpox (monkeypox). 2 September 2022. Available at: https://www.who.int/news-room/public-advice/protecting-yourself-from-monkeypox
- World Health Organization. Public advice for men who have sex with men on preventing mpox (monkeypox). 2 September 2022. Available at: https://www.who.int/news-room/public-advice/men-who-have-sex-with-men-preventing-monkeypox
- World Health Organization. Public health advice for sex workers on mpox. 18 September 2024. Available at: https://www.who.int/publications/m/item/public-health-advice-for-sex-workers-on-monkeypox
- World Health Organization. Public health advice on understanding, preventing and addressing stigma and discrimination related to mpox. 18 November 2024. Available at: https://www.who.int/publications/m/item/public-health-advice-on-understanding-preventing-and-addressing-stigma-and-discrimination-related-to-mpox
- World Health Organization. Public health advice on mpox and congregate settings: settings in which people live, stay or work in proximity. 20 March 2023. Available at: https://www.who.int/publications/m/item/public-health-advice-on-mpox-and-congregate-settings--settings-in-which-people-live--stay-or-work-in-proximity
- World Health Organization. Public health advice on mpox for people living in camps, refugee populations, internally displaced people and migrants. 14 October 2024. Available at: https://www.who.int/publications/m/item/public-health-advice-on-mpox-for-people-living-in-camps--refugee-populations--internally-displaced-people-and-migrants
- World Health Organization. Public health advice for people recovering from or caring for someone with mpox at home in low-resource settings. 19 December 2024. Available at: https://www.who.int/publications/m/item/public-health-advice-for-people-recovering-from-or-caring-for-someone-with-mpox-at-home-in-low-resource-settings
Citable reference: World Health Organization (5 December 2025). Disease Outbreak News; Broader transmission of clade Ib mpox - Global situation. Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2025-DON587
