This Medical Product Alert relates to confirmed falsified versions of ICLUSIG 15mg and ICLUSIG 45mg circulating in the WHO Region of Europe and the WHO Region of the Americas. Genuine ICLUSIG, the active pharmaceutical ingredient of which is Ponatinib Hydrochloride, is used to treat different forms of leukaemia.
On 15 January 2019, WHO was informed by health authorities in Switzerland that a local wholesaler had purchased packs of ICLUSIG 15mg : upon verification, the market authorization holder confirmed these packs as falsified. Further investigation confirmed that there are two versions of falsified ICLUSIG being traded globally, including via internet sales, detailed in the below table:
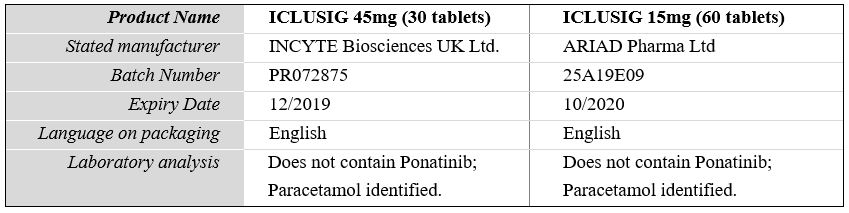
Laboratory analysis of ICLUSIG 15mg with batch number 25A19E09 has confirmed that the product does not contain Ponatinib and instead contains paracetamol.
Laboratory analysis of ICLUSIG 45mg with batch number PR072875 has confirmed that the product does not contain Ponatinib and instead contains paracetamol.
ICLUSIG is commercialized by different stakeholders in different parts of the world. The pharmaceutical companies TAKEDA and INCYTE are the genuine manufacturers / market authorization holders for ICLUSIG in the regions in which the above falsified versions have been discovered to date and they have both confirmed to WHO that:
- They did not manufacture or supply the above products, and
- The above batch numbers do not correspond to genuine manufacturing records.
Photographs are available below.
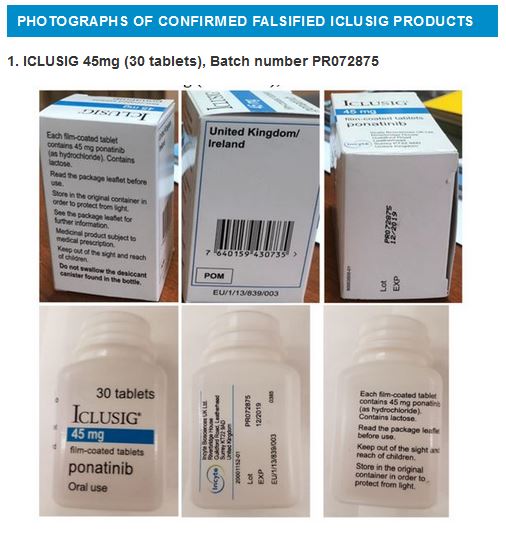
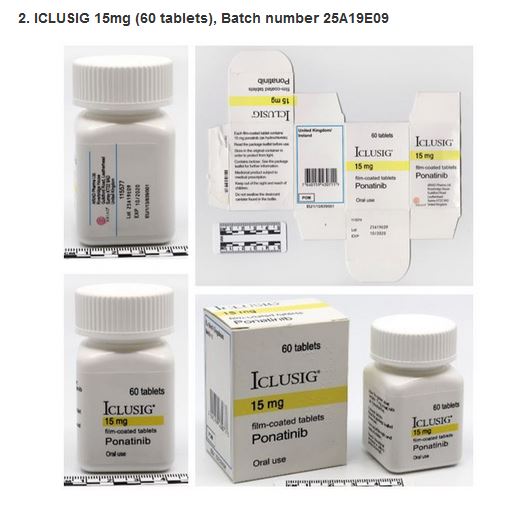
WHO requests increased vigilance within the supply chains of countries likely to be affected by these falsified products. Increased vigilance should include hospitals, clinics, health centres, wholesalers, distributors, pharmacies and any other suppliers of medical products.
If you are in possession of the above products, please do not use. If you have taken this falsified product, or if you suffer an adverse event or an unexpected lack of efficacy, please seek immediate advice from a qualified healthcare professional, and ensure they report the incident to your local Ministry of Health/National Medicines Regulatory Authorities/National Pharmacovigilance Centre.
All medical products must be obtained from authentic and reliable sources. Their authenticity and condition should be carefully checked. Seek advice from a healthcare professional in case of doubt.
National health authorities are asked to immediately notify WHO if these falsified products are discovered in their country. If you have any information concerning the manufacture, distribution, or supply of these products, please contact: rapidalert@who.int
Substandard and Falsified (SF) Medical ProductsAll WHO Drug Alerts are available at the following link: https://www.who.int/teams/regulation-prequalification/incidents-and-SF/full-list-of-who-medical-product-alerts
To sign up for WHO Medical Product Alerts, please visit: https://www.who.int/teams/regulation-prequalification/regulation-and-safety/rss
