Regulatory Systems Strengthening (RSS)
Related Tools and Initiatives
Publications
All →Roadmap for access to medicines, vaccines and health product 2019-2023: comprehensive support for access...
Equitable access to health products is a global priority, and the availability, accessibility, acceptability, and affordability of health products of...
The new 2030 development agenda and increasing globalization of health products development and supply have generated a need—and an opportunity—for...
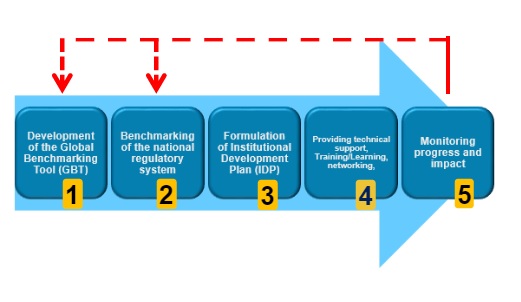
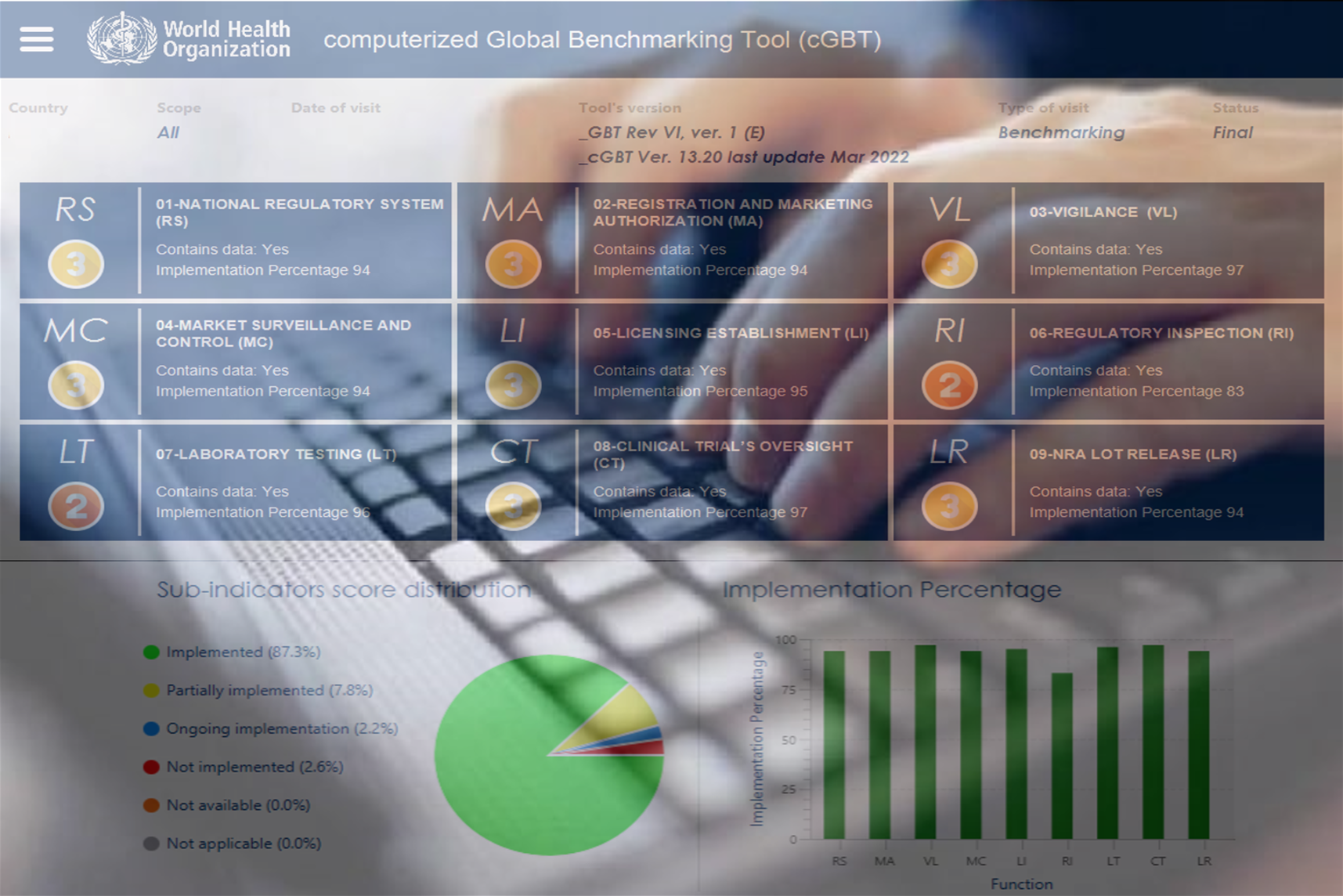
WHO Global Benchmarking Tool (GBT) for evaluation of national regulatory system of medical products:...
This manual is intended to provide clear operational guidance on the benchmarking of regulatory systems for medical products and the development of institutional development...
Global competency framework for regulators of medicines
The Global Competency Framework for Regulators of Medicines (GCF) provides a framework for best practices and general considerations aimed at harmonizing...
Manual for the performance evaluation of regulatory authorities seeking the designation as WHO-listed...
The Manual for the performance evaluation of regulatory authorities seeking the designation as WHO-listed authorities should be read in conjunction...

Operational guidance for evaluating and publicly designating regulatory authorities as WHO-listed...
The Operational guidance for evaluating and publicly designating regulatory authorities as WHO-listed authorities provides procedural information (processes,...
Implementing quality management systems in national regulatory authorities: Examples and practices
This document has been developed to assist NRAs with the practical implementation of the WHO guideline within their respective contexts. The document...
This policy on the evaluation and designation of regulatory authorities as WHO listed authorities was developed following broad public consultation and...
WHO Global Benchmarking Tool (GBT) for Evaluation of National Regulatory System of Medical Products -...
The World Health Organization (WHO) considers medical products and other health technologies one of the six building blocks of health systems. Unlike...
Guidance on developing a national deployment and vaccination plan for COVID-19 vaccines
This is an outdated version of this Interim Guidance which has since been updated. The latest version can be found here: https://www.who.int/publications/i/item/WHO-2019-nCoV-Vaccine-deployment-2021.1-eng The Guidance...