
2.2 Diagnostic testing
An essential step in the pathway of tuberculosis (TB) care is rapid and accurate testing. Since 2011, rapid molecular tests that are highly specific and sensitive have revolutionized the TB diagnostic landscape, which previously relied upon more traditional microscopy and culture methods.
People diagnosed with TB using rapid molecular tests recommended by WHO (1), lateral flow urine lipoarabinomannan (LF-LAM) assays, sputum smear microscopy or culture are defined as “bacteriologically confirmed” cases of TB (2). The microbiological detection of TB is critical because it allows people to be correctly diagnosed and started on the most effective treatment regimen as early as possible. People diagnosed with TB in the absence of bacteriological confirmation are classified as “clinically diagnosed” cases of TB.
Bacteriological confirmation of TB is necessary to test for resistance to anti-TB drugs. Such testing can be done using rapid molecular tests, phenotypic susceptibility testing or (at reference-level laboratories) genetic sequencing.
A global total of 7.5 million people were newly diagnosed with TB and notified as a TB case in 2022; of these 6.2 million (83%) had pulmonary TB (Table 2.1.1 of Section 2.1). Worldwide, the percentage of people diagnosed with TB based on bacteriological confirmation improved between 2018 and 2021, from 55% to 63%; it remained at 63% in 2022 (Fig. 2.2.1). Among the six WHO regions, the highest percentage in 2022 was in the Region of the Americas (79%). Improvements to comparable levels are required in other regions.
In 2022, there was considerable country variation in the proportion of people diagnosed with a new episode of pulmonary TB who were bacteriologically confirmed (Fig. 2.2.2).
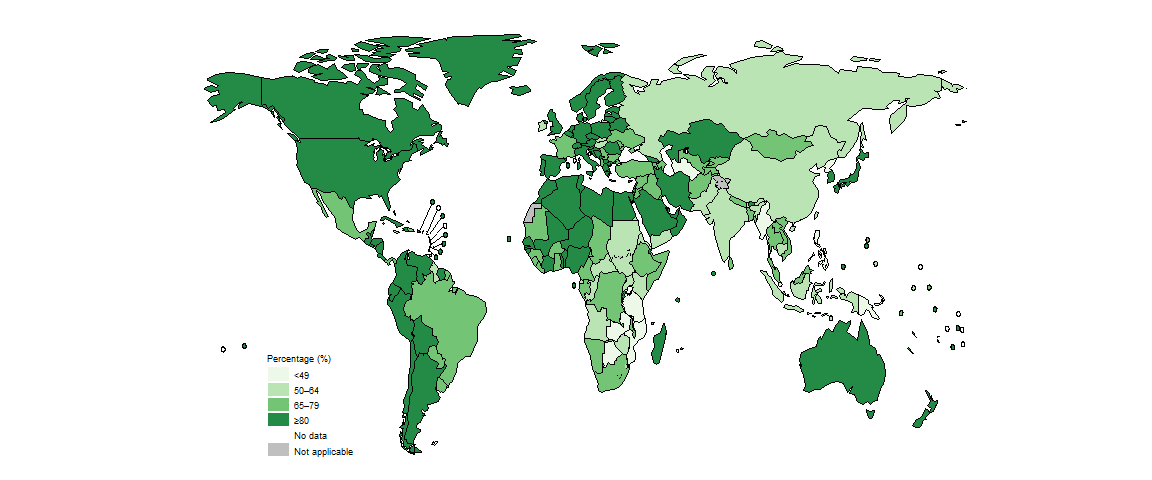
In general in 2022, levels of bacteriological confirmation were highest in high-income countries (median, 89%), where there is wide access to the most sensitive diagnostic tests, and lowest in low-income countries (median, 71%) (Fig. 2.2.3). Over-reliance on direct sputum smear microscopy is inherently associated with a relatively high proportion of pulmonary TB cases that are clinically diagnosed, as opposed to bacteriologically confirmed.
In the 30 high TB burden countries (Fig. 2.2.4), variation in the proportion of people diagnosed with pulmonary TB who were bacteriologically confirmed likely reflects differences in diagnostic and reporting practices. The countries with relatively high levels of bacteriological confirmation in 2022 (75% or above) were Bangladesh, Brazil, Liberia, Mongolia, Namibia, Nigeria and Viet Nam.
There is clear scope for improvement in most other high TB burden countries. This is particularly needed in the Democratic People’s Republic of Korea, Mozambique, Myanmar, Pakistan, Papua New Guinea, the Philippines, the United Republic of Tanzania and Zambia, where levels of bacteriological confirmation remained around or below 50% in 2022. Such levels of bacteriological confirmation show overdependence on clinical diagnosis of TB and potentially over-diagnosis. When the proportion of people diagnosed with pulmonary TB based on bacteriological confirmation falls to around or below 50%, a review of the diagnostic tests in use and the validity of clinical diagnoses is warranted (e.g. via a clinical audit).
Globally in 2022, a WHO-recommended rapid diagnostic test (WRD) was used as the initial diagnostic test for 47% (3.5 million) of the 7.5 million people newly diagnosed with TB in 2022, up from 38% (out of a total of 6.4 million) in 2021 and 33% (out of a total of 5.8 million) in 2020 (Fig. 2.2.5). Among WHO regions, the best level of coverage was achieved in the European Region (77%); the lowest coverage was in the South-East Asia Region (39%).
Use of WRDs varied substantially among countries in 2022 (Fig. 2.2.6).
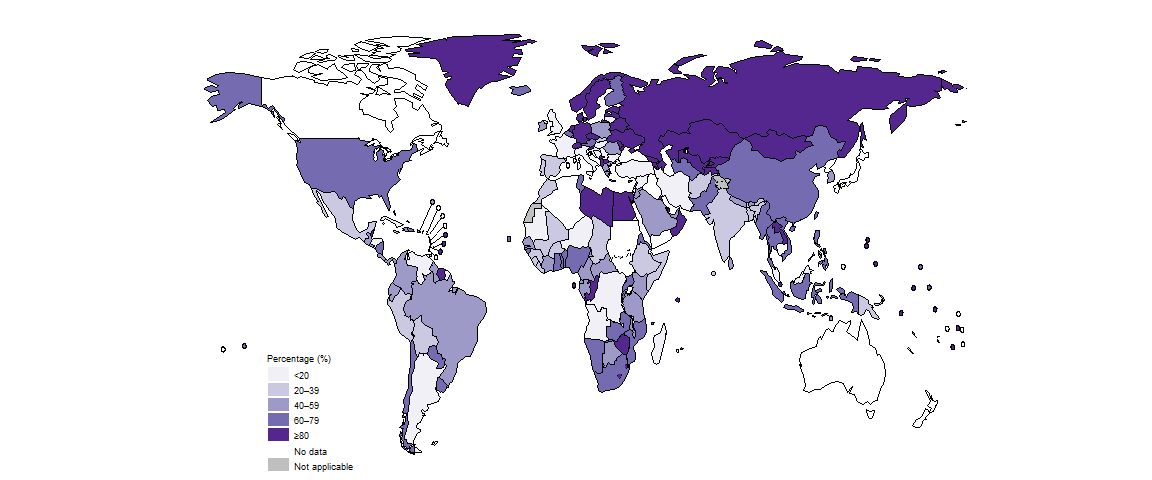
Among the 30 high TB burden countries, those with the high proportions (≥80%) of people diagnosed with TB who were initially tested with a WRD included Congo, Lesotho and Mongolia (Fig. 2.2.7).
Among the 49 countries in one of the three global lists of high burden countries (for TB, HIV-associated TB and MDR/RR-TB) being used by WHO in the period 2021–2025 (Annex 3), 31 reported that a WRD had been used as the initial test for more than half of their notified TB cases in 2022, up from 27 in 2021 and 21 in 2020.
In 2022, the proportion of TB diagnostic sites with access to WRDs varied considerably by country (Fig. 2.2.8). Only six of the 30 high TB burden countries reported that more than 50% of their TB diagnostic sites had access to WRDs. Expanding access to TB diagnosis using rapid tests should be a top priority in many countries. Globally, the median was 33% (interquartile range (IQR): 13–75%).

A second indicator that can be used to assess access to rapid testing for TB is the number of WRDs used per capita. In 2021 and 2022, there was substantial variation among the 30 high TB burden countries (Fig. 2.2.9). The five countries that exceeded 1000 tests per 100 000 population were all in the African Region: Lesotho, Namibia, South Africa, Uganda and Zambia. In most of the 30 high TB burden countries, the number per capita increased between 2021 and 2022.
The percentage of people initially tested with a WRD who had a positive test result provides an indication of the level of case-finding efforts (Fig. 2.2.10). A low percentage suggests a lack of precision in deciding who to test, while a high percentage suggests suboptimal efforts to detect people with TB. In 2021 and 2022, there was considerable variation among the 30 high TB burden countries and WHO regions.
The number of WRDs used per person notified as a TB case also provides an indication of the level of diagnostic effort based on rapid tests. In 2021 and 2022, the number of rapid tests per notified case varied widely in the 30 high TB burden countries, from less than two in 11 countries to a high of 12 in South Africa (Fig. 2.2.11). In most of these countries, the number of tests per case increased between 2021 and 2022.
Of the 7.5 million people newly diagnosed with TB globally in 2022, 80% had a documented HIV test result, a further improvement from 76% in 2021 (Fig. 2.2.12). At regional level, the highest percentages were achieved in the WHO African and European regions.
b Countries were excluded if the number of people with documented HIV status was not reported to WHO.
There was considerable variation at national level (Fig. 2.2.13). In 97 countries and areas, at least 90% of people diagnosed with TB in 2022 knew their HIV status; this included 32/47 countries in the African Region, where the burden of HIV-associated TB is highest. In the other five WHO regions, the percentage was above 50% in most countries. In 2022, there were 16 countries in which less than half of the people diagnosed with TB knew their HIV status: Argentina, Bangladesh, Bosnia and Herzegovina, Canada, Gabon, Hungary, Ireland, Japan, North Macedonia, Serbia, Solomon Islands, Sudan, Syrian Arab Republic, Tunisia, Venezuela (Bolivarian Republic of) and Yemen.
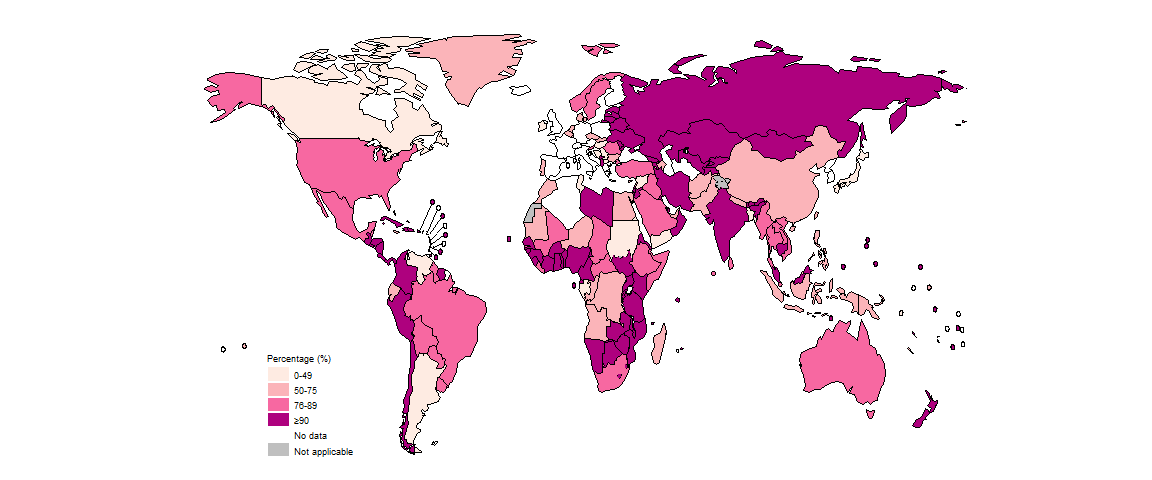
Worldwide in 2022, a total of 426 958 cases of TB among people living with HIV were notified (Table 2.1.1 of Section 2.1), equivalent to 7.3% of the 5.8 million people diagnosed with TB who had an HIV test result. Overall, the percentage of people diagnosed with TB who had an HIV-positive test result has fallen globally over the past ten years.
Bacteriological confirmation of TB is necessary to test for drug-resistant TB. Globally in 2022, 73% of people with bacteriologically confirmed TB were tested for resistance to rifampicin (the most effective first-line anti-TB drug), up from 69% in 2021 (Fig. 2.2.14). There were also improvements in five of the six WHO regions; the exception was the WHO European Region.
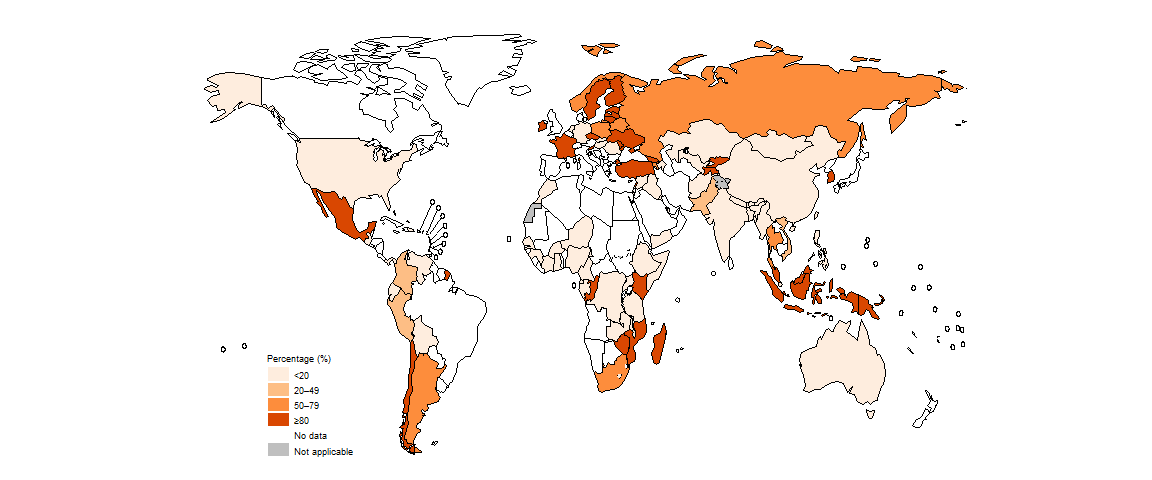
In 2022, there was considerable variation in the coverage of testing for rifampicin-resistant TB (RR-TB) among countries (Fig. 2.2.15). Of the 30 countries (see Annex 3) that WHO has defined as high burden for multidrug-resistant/RR-TB (MDR/RR-TB; MDR-TB is defined as resistance to both rifampicin and isoniazid), 19 reached a coverage of at least 80% in 2022: Angola, Azerbaijan, Belarus, China, Kazakhstan, Kyrgyzstan, Mongolia, Mozambique, Myanmar, Nigeria, the Philippines, the Republic of Moldova, the Russian Federation, South Africa, Tajikistan, Ukraine, Uzbekistan, Viet Nam and Zimbabwe.

Among people tested for RR-TB, a total of 149 511 cases of MDR/RR-TB and 27 075 cases of pre-extensively drug-resistant TB (pre-XDR-TB) or XDR-TB were detected (Table 2.1.1), for a combined total of 176 586. This was a small increase (5.6%) from a combined total of 167 211 in 2021, and smaller than the 16% increase in the number of people diagnosed and reported with TB between 2021 and 2022 (Section 2.1). Since 2021, pre-XDR-TB is defined as MDR/RR-TB plus resistance to any fluoroquinolone (3).
The global and regional coverage of testing for susceptibility to fluoroquinolones, which is necessary to determine the most appropriate treatment regimen for people with RR-TB (4), is lower than the coverage of testing for RR-TB (Fig. 2.2.16). Among WHO regions in 2022, coverage was highest in the WHO European Region (80%) and lowest in the Western Pacific Region (28%).
There is considerable country variation in the proportion of people diagnosed with RR-TB who were tested for susceptibility to fluoroquinolones (Fig. 2.2.17).

Bedaquiline is recommended by WHO as part of treatment regimens for people with MDR/RR-TB and pre-XDR-TB (4). In 2022, the percentage of people with pre-XDR-TB who were tested for susceptibility to bedaquiline remained low in most parts of the world (Fig. 2.2.18).

Linezolid is also recommended by WHO as part of treatment regimens for MDR/RR-TB as well as pre-XDR-TB (4). In 2022, the percentage of people with pre-XDR-TB who were tested for susceptibility to linezolid remained low in most parts of the world (Fig. 2.2.19).
Further country-specific details about diagnostic testing for TB, HIV-associated TB and anti-TB drug resistance are available in the Global tuberculosis report app and country profiles.
Data shown on this webpage are as of 21 July 2023 (see Annex 2 of the main report for more details).
References
WHO consolidated guidelines on tuberculosis. Module 3: Diagnosis – rapid diagnostics for tuberculosis detection 2021 update. Geneva: World Health Organization; 2021 (https://iris.who.int/handle/10665/342331).
Definitions and reporting framework for tuberculosis – 2013 revision (updated December 2014 and January 2020) (WHO/HTM/TB/2012.2). Geneva: World Health Organization; 2013 (https://iris.who.int/handle/10665/79199).
Meeting report of the WHO expert consultation on the definition of extensively drug-resistant tuberculosis, 27-29 October 2020. Geneva: World Health Organization; 2021 (https://iris.who.int/handle/10665/338776).
WHO consolidated guidelines on tuberculosis. Module 4: Treatment – drug-resistant tuberculosis treatment. Geneva: World Health Organization; 2020 (https://iris.who.int/handle/10665/332397).
