Marker classification and evidence review
Marker classification framework
Different types of evidence were considered to determine the association between specific genetic alterations and antimalarial drug resistance. Alterations that met the domain-specific thresholds were considered for inclusion, while those that did not meet minimum criteria in any domain were excluded.
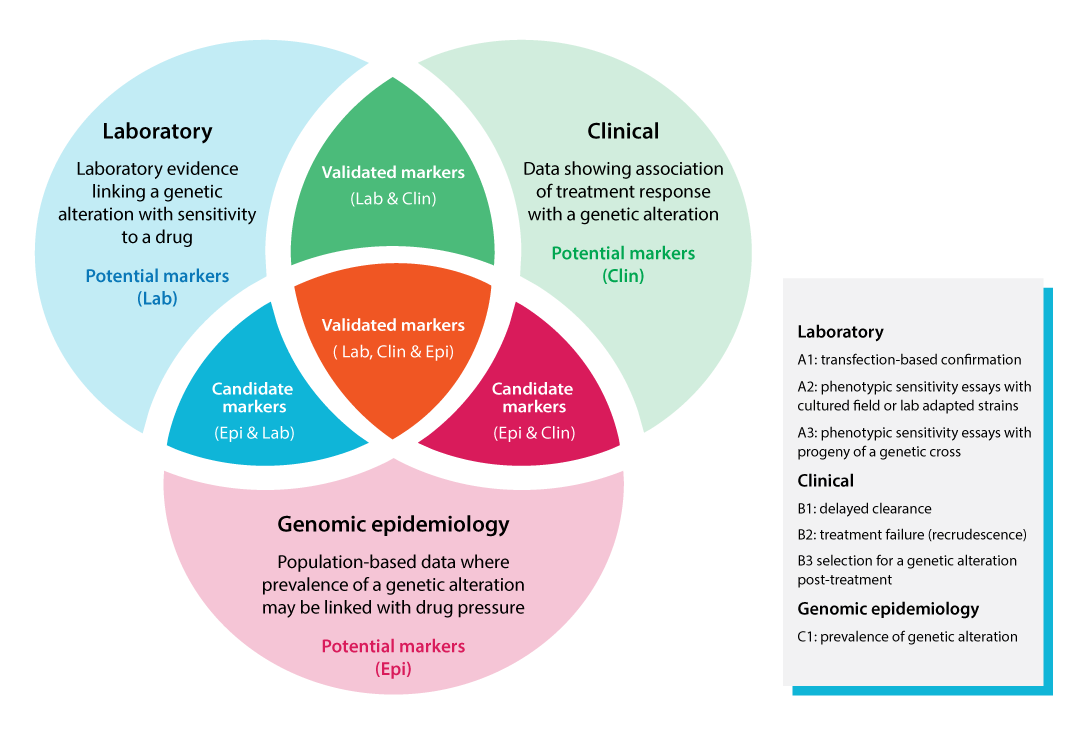
Based on a combined assessment of evidence across the three domains, each genetic alteration was classified as a potential, candidate, or validated marker of antimalarial drug resistance. The Venn diagram illustrates the classification framework, integrating laboratory, clinical, and genetic epidemiology data to define the three categories of molecular markers, reflecting the level of confidence in the evidence supporting their association with drug resistance.
Marker classification framework
|
Evaluation framework
Molecular markers were evaluated using three domains of evidence encompassing different types of data: laboratory (A1-A3), clinical (B1-B3), and genetic epidemiology (C1). The data represented in the evidence reviewed and their respective strengths and limitations are outlined below (for further details, please refer to the full document on Methods and thresholds).
Laboratory evidence provides direct insight into how genetic alterations affect parasite susceptibility under controlled in vitro conditions. The strongest evidence comes from transfection experiments (A1), which introduce specific alterations into a defined genetic background to confirm their effect on drug susceptibility. Other data sources include drug susceptibility assays on field isolates or culture-adapted laboratory strains (A2), or on progeny from genetic crosses (A3). While less conclusive, these studies still contribute valuable information on resistance mechanisms and potential evolution.
Clinical evidence links genetic alterations to patient outcomes by analysing delayed parasite clearance (B1), treatment failure (recrudescence) (B2), or selection of variants after treatment in a patient (B3). Clinical evidence is central, as therapeutic efficacy in patients remains the foundation for guiding malaria treatment policy. However, interpreting clinical outcomes can be complex, and causal attribution to a specific genetic alteration remains challenging. This is particularly true for artemisinin-based combination therapies (ACTs), where the effects of both components must be considered. Factors such as drug absorption, metabolism, and host immunity also influence clinical outcomes.
Genetic epidemiological evidence reflects parasite population dynamics and indicates the transmission or spreading potential of a genetic alteration. A genetic alteration observed at significant prevalence may indicate its potential to spread under current conditions, including existing treatment practices and drug pressure (C1). However, establishing causality is challenging, particularly for combination therapies, and genetic epidemiology should always be interpreted with caution, serving primarily as supporting evidence alongside laboratory and clinical data.
Evidence reviewed
A total of 3 441 publications, published before May 2025, pertaining to antimalarial drug resistance in P. falciparum were identified in the initial search. Of these, 863 articles contained potentially relevant evidence and were included in the initial expert review. Laboratory, clinical, and genetic epidemiological evidence corresponding to genetic alterations reported in these articles were evaluated against established thresholds, and classifications were refined based on expert assessment of study quality and methodology. Articles presenting evidence that did not meet the predefined criteria, such as weak or non-significant associations, very small sample sizes, or insufficient supporting data were excluded. The current compendium for P. falciparum references 283 articles, which together form the evidence base for the markers summarized in the detailed tables. Content for P. vivax is under development and will be added in an upcoming release.