WHO/Y. Shimizu
On the fourth day of its annual meeting, the WHO Regional Committee for the Western Pacific updated participants on progress made in the Region with regard to reducing child deaths, preparing Member States to respond to antimicrobial resistance (AMR), and improving access to quality medicines.
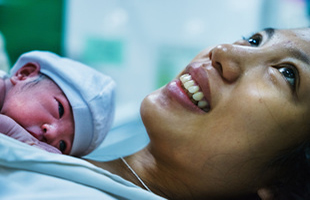
Healthy newborn infants
While significant progress has been made in reducing child deaths in the Region, newborn deaths remain unacceptably high. In 2013, newborn deaths accounted for more than 50% of deaths of children aged 5 and below, mainly because of inappropriate clinical practices and gaps in health systems.
In response, the Regional Committee in 2013 endorsed the Action Plan for Healthy Newborn Infants in the Western Pacific Region (2014–2020), which focuses on practices in health-care facilities, where close to 100% of births occur in the Region.
The action plan outlines an approach for implementing and scaling up quality Early Essential Newborn Care (EENC) by: (1) ensuring consistent adoption and implementation of EENC; (2) improving political and social support to ensure an enabling environment; (3) ensuring availability of, access to and the use of skilled birth attendants and essential maternal and newborn commodities; (4) engaging and mobilizing communities to increase demand; and (5) improving the quality and availability of perinatal information.
EENC has been introduced to more than 27 700 health providers in health facilities, including more than 80% of national hospitals in the Lao People's Democratic Republic, Mongolia, Papua New Guinea and Solomon Islands, as well as all provincial-level hospitals in Cambodia, Mongolia and the Philippines.
As a result, immediate newborn care practices have drastically improved in Cambodia, China, the Lao People's Democratic Republic, Mongolia, Papua New Guinea, the Philippines, Solomon Islands and Viet Nam. In participating health facilities, 72% of newborn infants are now placed in immediate skin-to-skin contact with mothers. Nearly half remain in skin-to-skin contact through the first breastfeeding, and four out of five are exclusively breastfed in the first days of life.
However, only 7% of preterm newborn infants receive Kangaroo Mother Care, which generally includes skin-to-skin contact, feeding with breast milk, and additional support for mother and baby. Newborn mortality declined in Cambodia from 27 to 18 deaths per 1000 live births between 2010 and 2014. In the Philippines, the figure went from 16 to 13 deaths per 1000 live births between 2008 and 2013.
Guidance to tackle antimicrobial resistance
In 2014, the Antimicrobial Resistance: Global Report on Surveillance highlighted alarming rates of resistance in common bacteria that cause health-care-associated and community-acquired infections in the Western Pacific Region.
To address this, the sixty-fifth session of the Regional Committee for the Western Pacific endorsed the Action Agenda for Antimicrobial Resistance in the Western Pacific Region to (1) develop and implement national plans to contain AMR; (2) improve AMR surveillance and the monitoring of antimicrobial use; and (3) strengthen the health system response to contain AMR.
Significant progress has been made towards the implementation of the action agenda. Six countries (Australia, Cambodia, Fiji, Japan, the Philippines and Viet Nam) have launched comprehensive national AMR action plans. Six more countries are developing national action plans. Political commitment to AMR was confirmed in 2015 when the World Health Assembly called on all countries to develop national AMR action plans by May 2017.
This commitment was reaffirmed in 2016 when ministers of health from 11 countries from the WHO South-East Asia and Western Pacific regions signed the Communiqué of Tokyo Meeting of Health Ministers on Antimicrobial Resistance in Asia. AMR also figured prominently on the agendas of high-level meetings such as the United Nations General Assembly, the ASEAN meeting, etc.
The first World Antibiotic Awareness Week was celebrated in 2015 to increase understanding of AMR and the need for responsible use of antimicrobials. Twenty-one countries in the Region held national campaign activities targeting policy-makers, health-care professionals and the general public. Planning is already in progress for the 2016 campaign, which will adopt a multisectoral and collaborative approach to disseminate information. This will involve WHO, the Food and Agriculture Organization of the United Nations (FAO) and the World Organisation for Animal Health (OIE) coming together to create awareness on the impacts of antibiotics on humans, animals and agriculture.
Making quality medicines accessible to all
Member States have achieved the goal of improving access to quality, safe, efficacious and affordable medical products by focusing on three key areas: (1) policy and access to essential medicines; (2) regulation and quality assurance; and (3) the rational selection and use of medicines.
The Regional Framework for Action on Access to Essential Medicines in the Western Pacific (2011–2016) has provided solid technical guidance to assist countries in developing national medicines policies, particularly in China, Malaysia and Viet Nam.
WHO also supported the development, review and implementation of national medicines policies that promote equitable and sustainable access to essential medicines in Brunei Darussalam, Cambodia, China, Cook Islands, Fiji, Kiribati, the Lao People's Democratic Republic, the Marshall Islands, Malaysia, the Federated States of Micronesia, Mongolia, Nauru, Palau, Papua New Guinea, the Philippines, Samoa, Solomon Islands, Tonga, Tuvalu, Vanuatu and Viet Nam.
Regulatory system capacity was strengthened to ensure the quality and safety of medicines, vaccines and other health technologies in Cambodia, China, Fiji, the Lao People's Democratic Republic, Malaysia, Mongolia, Papua New Guinea, the Philippines and Viet Nam. Specific areas strengthened included medical product registration, inspections, good manufacturing practices, reviews of legislation and pharmacovigilance. China and Viet Nam were certified as having functional vaccine regulatory systems in 2011 and 2015, respectively.
Member States have also received training on detection and reporting of counterfeit medical products as part of the WHO global surveillance and rapid alert system.
To improve access to medicine for children, the World Health Assembly in May 2016 endorsed a resolution to promote innovation and access to quality, safe, efficacious and affordable medicines for children. WHO will assist Member States in accelerating implementation.




