Laboratory Testing
Introduction
The WHO Global RSV Surveillance (WHO-GRSVS) is an integrated laboratory and epidemiological surveillance that was established to standardize surveillance and monitor the evolving trends of the Human Respiratory Syncytial Virus (hRSV)[1].
Human Respiratory Syncytial Virus is a negatively stranded, enveloped RNA virus and belongs to the Orthopneumovirus genus of the Pneumoviridae family. It is divided into two antigenic groups (subgroups) hRSV A and hRSV B.[2] The genome is 15 kb long with 10 gene transcripts encoding 11 proteins of which the G glycoprotein and the fusion (F) glycoprotein are major surface proteins that function in attachment. Antigenic groups can be further divided into 16 RSV-A and 22 RSV-B genotypes based on the sequence variability of the second hypervariable region (HVR2) of the distal third region of the G gene. [3] Figure 1
Genomic surveillance of the hRSV at the global level is important to improve the understanding of; antigenic and genetic variability of circulating strains, implications on seasonality and outbreaks, transmission dynamics, monoclonal therapies and vaccine development.
Variability in the F protein has been shown to correlate with disease severity and may have implications for effectiveness of monoclonal drugs and vaccines development. In addition, genetic insertions in the HVR2 of the G protein of RSV-A and RSV-B has resulted in changes in the antigenicity which enabled viral escape from the host immunity. Such examples included the emergence of RSV-A ON1 and RSV-B BA which subsequently became the more prevalent genotypes globally.[1] WHO Strategy for Global Respiratory Syncytial Virus Surveillance Project based on the Influenza Platform. Revised based on outcomes of the pilot phase. Geneva, Switzerland. 2019.
[2] Mas, V., Nair, H., Campbell, H., Melero, J. A., & Williams, T. C. (2018). Antigenic and sequence variability of the human respiratory syncytial virus F glycoprotein compared to related viruses in a comprehensive dataset. Vaccine, 36(45), 6660-6673. https://www.sciencedirect.com/science/article/pii/S0264410X18313252
[3] Respiratory syncytial virus genotypes NA1, ON1, and BA9 are prevalent in Thailand, 2012–2015
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5661434/
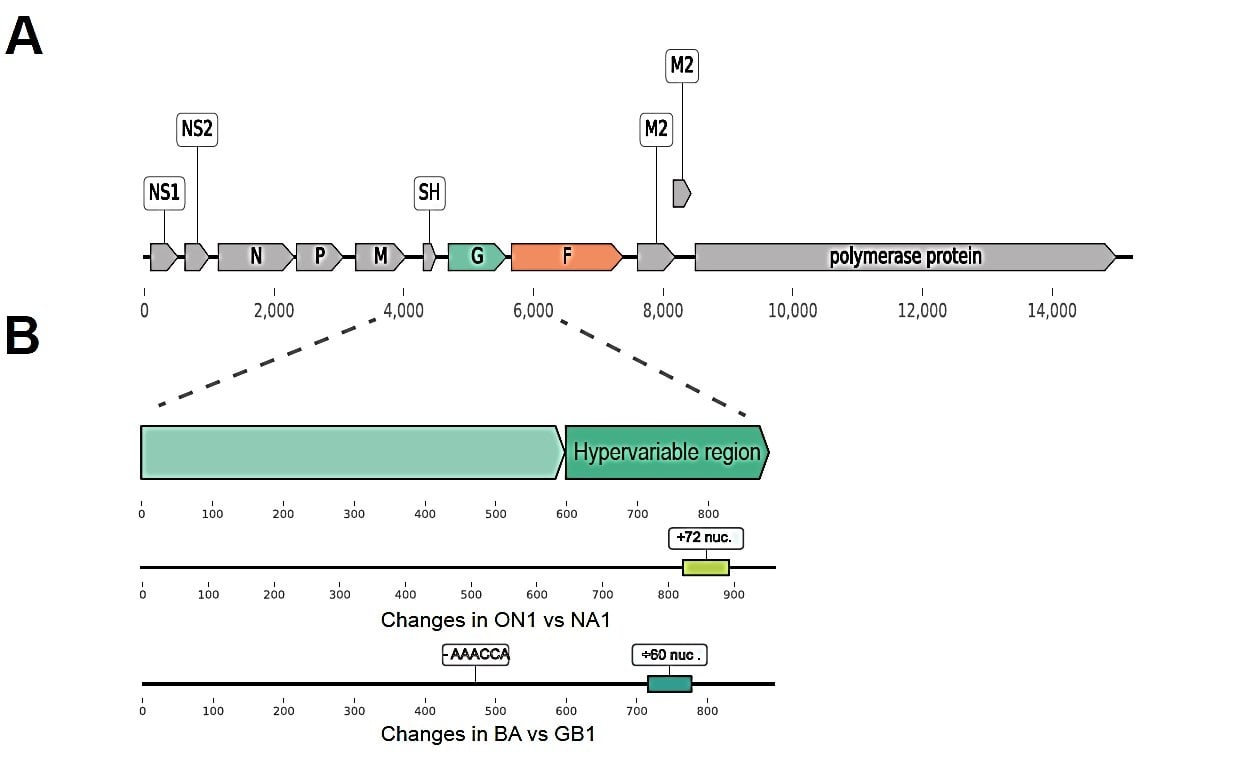
Figure 1. Human RSV genome showing; (a) Changes in ON1 vs NA1 genotype- the 72-nucleotide duplication in the HVR2 of RSV-A (ON1) genotype. The ON1 genotype emerged in Canada 2012 and is now detected in Europe, Africa, Asia, and the Americas. (b) Changes in BA vs NA1 genotype showing the 60-nucleotide duplication in the HVR2. BA was first identified in Buenos Aires in 2003 and has since emerged as the prevalent genotype globally.
Improving RSV molecular detection, typing and sequencing capacity in participating laboratories of the WHO Global RSV Surveillance- Phase 2
Following the successful implementation of the WHO Global RSV Surveillance as a Pilot in 14 countries from 2015 to 2018, RSV surveillance was continued as Phase 2 and expanded in April 2019 to a total of 25 laboratories in 23 countries of the 6 WHO Regions. Whereas the Pilot focused on detection only of RSV, Phase 2 will increase laboratory capacity to include typing of RSV A and RSV B and genome sequencing (partial and whole).
WHO-GRSVS Laboratories are National Influenza Centres (NICs) or National Laboratories with RSV molecular testing capacities, as demonstrated by successful ongoing participation in the WHO EQA for the Molecular Detection of Influenza and in WHO acknowledged RSV External Quality Assessments (e.g. the US CDC RSV Proficiency Test and/or the WHO RSV EQA).
RSV Reference Laboratories (RSV RL) of the WHO-GRSVS are laboratories with established RSV diagnostic and research capacity which function under the WHO Terms of Reference for RSV Reference Laboratories[1]. Participating national WHO-GRSVS laboratories function in collaboration with RSV Reference Laboratories under the coordination of the WHO Global Influenza Program.
List of RSV Reference Laboratories:
- The Gastroenteritis and Respiratory Viruses Laboratory Branch at the CDC, Atlanta
- The National Institute for Communicable Diseases, Johannesburg, South Africa.
- Public Health England, Colindale, London
- The WHO Collaborating Centre for Reference and Research on Influenza, The Victorian Infectious Diseases Reference Laboratory (VIDRL), Australia
List of RSV Pilot Laboratories (updated January 2021)
Summary objectives of Phase 2
Participating laboratories in Phase 2 will detect and type RSV. Laboratories will work closely with Reference Laboratories in the standardization of typing methodologies and sequencing of viruses. Reference laboratories will take the lead in establishing sequencing protocols. Sequencing will be performed on RSV positive samples collected during the Pilot and on viruses from RSV positive specimens from Phase 2.
Key laboratory objectives of Phase 2 are to;
- standardize and implement laboratory protocols for the molecular detection, typing and sequencing of RSV.
- develop and implement updated RSV external quality assurance tests for the molecular detection and typing of RSV.
- describe the molecular epidemiology of RSV at the genomic level and the implications of genetic variability on the monoclonal antibodies and RSV vaccines.
- enrich the globally representative bioinformatic data in publicly accessible database(s) and therefore support an improved understanding of the evolutionary diversity of RSV genetics.
[1] WHO Strategy for Global Respiratory Syncytial Virus Surveillance Project based on the Influenza Platform. Revised based on outcomes of the pilot phase. Geneva, Switzerland. 2019.
