Global Respiratory Syncytial Virus Surveillance
Respiratory Syncytial Virus (RSV) is a leading cause of hospitalization due to acute lower respiratory infection especially in infants and young children. Currently available options (Palivizumab) for preventing and treating RSV are limited to select populations in high-resource settings. Fortunately, several vaccine candidates are now in the human testing phase targeting young children, older adults and pregnant women, and an effective safe vaccine is likely to be available in the near future.
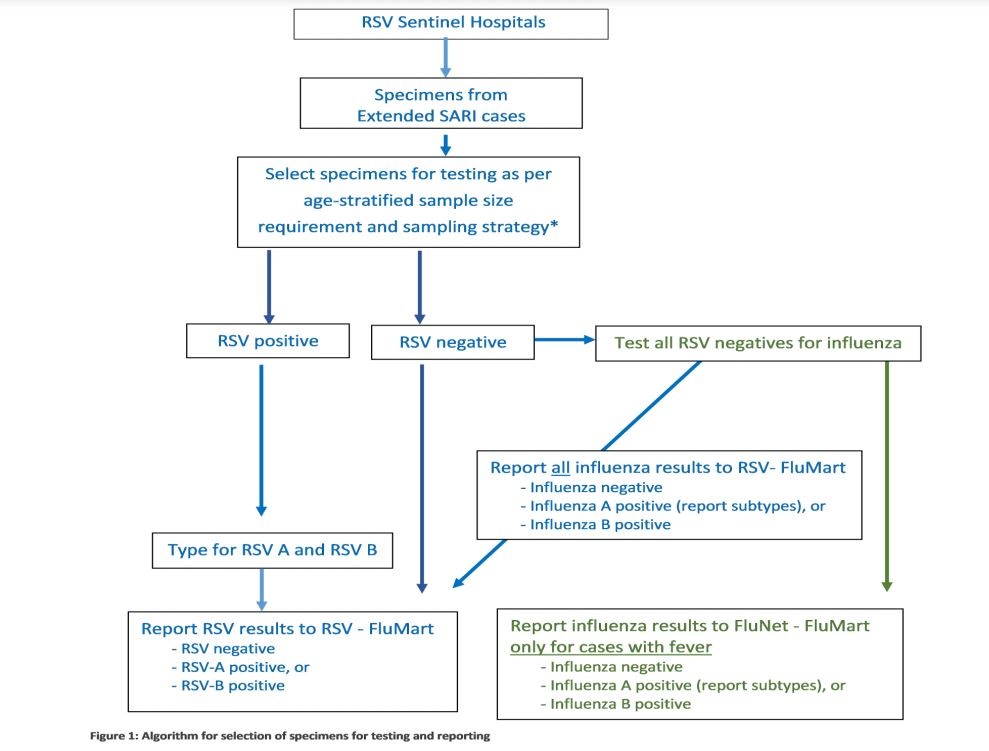
The WHO Global Influenza Programme with support from the Bill & Melinda Gates Foundation, piloted a RSV surveillance strategy Phase I based on the Global Influenza Surveillance and Response System (GISRS) in 14 countries in order to standardized RSV surveillance and provide evidence to support Public Health and to inform RSV vaccination policy. The output from the pilot project establishes a solid RSV surveillance strategy with evidence-based standards and a tested mechanism for RSV surveillance based on the GISRS. The phase II has started with a 3-year extension phase (Nov 2018 – Oct 2021) aims to consolidate the achievements of the original investment.