This page contains a repository of immunization supply chain guidance and tools produced by WHO and is organized into the following topics:
Supply chain publications
International shipping of vaccines is the first leg of the complex journey that vaccines undertake to reach the end users in a country. Particular challenges...
Some of the earliest COVID-19 vaccines deployed during the pandemic had a shorter shelf life at +2°C to +8°C. Therefore, transporting these vaccines...
Vaccine carriers protect vaccine potency during immunization sessions and transport to outreach sites. This guidance document is intended to help inform...
Other languages:ArabicRussian
Cold room temperature mapping studies
A cold room temperature mapping study establishes the temperature distribution in cold rooms – the largest vaccine storage equipment within a country’s...
Temperature monitoring study: a fully documented process to detect weaknesses in the supply chain
Studies in both industrialized and developing countries have revealed that vaccines are commonly exposed to damaging temperatures, especially exposure...
Solar direct-drive (SDD) refrigerators and freezers can be a good option for vaccine storage in areas without reliable electricity, and many models...
WHO guideline on the use of safety-engineered syringes for intramuscular, intradermal and subcutaneous...
Injections are one of the most common health care procedures. Every year at least 16 billion injections are administered worldwide. The vast majority –...
Introducing solar-powered vaccine refrigerator and freezer systems: a guide for managers in national...
This document provides managers in national immunization programmes with guidance on how to implement successful solar-powered vaccine refrigerator and...
WHO guidance note: Vaccine diluents, revision 2015
A vaccine diluent is the liquid mixed with a lyophilized (freeze-dried) vaccine in order to reconstitute the lyophilized vaccine and provide the final...
This revision to the multi-dose vial policy provides updated guidance on how to handle all opened multi-dose vials of WHO pre-qualified vaccines. It outlines...
Immunization supply chain and logistics - A neglected but essential system for national immunization...
In this Call-to-Action, the WHO Immunization Practices Advisory Committee (IPAC) advocates greater action by both national programmes and the global immunization...
Most countries now have well-established immunization services and a network of vaccine stores. This infrastructure must be capable of responding to change. There...
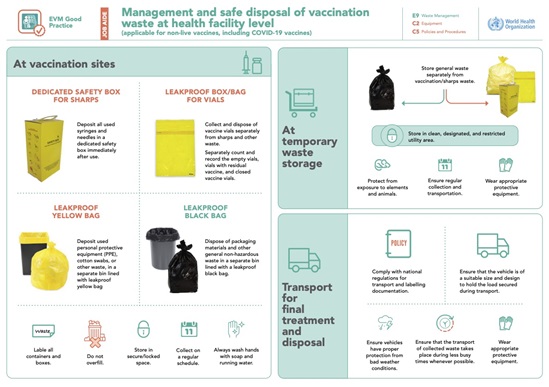
EVM Good Practice

Vaccine Management Handbook
The Vaccine Management Handbook, a component of the EVM Initiative, has been written for decision-makers at national and subnational levels; its purpose is to provide technical advice on key topics related to immunization logistics to help countries develop and refine national policies. For more detailed guidance on specific operational activities, refer to the EVM Standard Operating Procedures (SOPs).
Pour la version française, cliquez sur "Read More".
Vaccine Management Handbook: How to Develop a Repair and Maintenance System for Cold Chain Equipment
This module of the WHO Vaccine Management Handbook (VMH) introduces the policy, technical, material, budget and management requirements of an effective...
Vaccine Management Handbook: How to calculate Vaccine Volumes and Cold Chain Capacity Requirements
This module of the WHO Vaccine Management Handbook describes how to calculate vaccine volumes and evaluate the cold chain capacity requirements of a...
This module of the WHO Vaccine Management Handbook (VMH) focuses on temperature monitoring and provides updated implementation guidance on vaccine vial...
Vaccine Management Handbook: How to use passive containers and coolant-packs for vaccine transport and...
This module of the WHO Vaccine Management Handbook (VMH) provides guidance on how to develop a transport strategy that minimizes the risk of exposure to...
WHO-UNICEF joint statements
Acceptance of available traditional vaccine supply with reduced shelf-life
Temperature-sensitive health products in the Expanded Programme on Immunization cold chain
Appropriate storage and management of oxytocin – a key commodity for maternal health
Vaccine vial monitor
VVMs (vaccine vial monitors) are small indicators that adhere to vaccine vials and change colour as the vaccine is exposed to cumulative heat, letting health workers know whether the vaccine has exceeded a pre-set limit beyond which the vaccine should not be used.
The following infographic summarizes how health workers can use VVMs to decide whether or not to use a vaccine vial. It presents VVM colour change as a continuous progression, rather than as four distinct stages, and can be included in guidance and training materials.
Additional resources related to VVMs can be found on the VVM page of the TechNet-21.org website.
The VVM infographic is available in five languages.