Priority Area 2: Evidence-informed policy guiding comprehensive and coordinated hepatitis action
National action plan and disease burden estimates
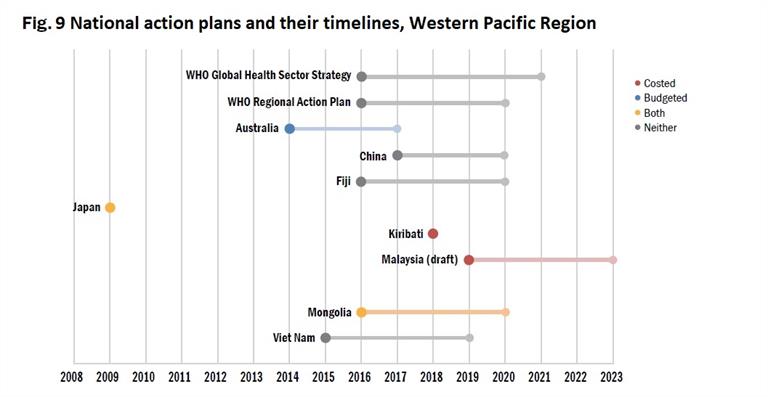
Eleven Member States have published comprehensive national action plans against viral hepatitis, and six have plans drafted or in progress. However, only two of the national action plans have been costed and funded (Fig. 9). These plans share similarities, with their focus on prevention and control of viral hepatitis and specific components centred on raising awareness, surveillance, vaccination, prevention of MTCT, prevention of transmission in health-care settings, testing and treatment, and coinfection with HIV.
Country-owned disease burden estimates for HBV or HCV have been conducted in Australia, Cambodia, China, Fiji, Hong Kong SAR (China), Japan, Kiribati, Macao SAR (China), Malaysia, Mongolia, New Zealand, the Philippines, the Republic of Korea, Singapore, Solomon Islands and Viet Nam. These estimates are important for establishing the current situation of the epidemic, monitoring the progress of national action plans and mobilizing resources.
Mobilizing resources for action: towards sustainable financing for hepatitis care
Many countries have a basic health insurance benefit package in place, and free HBV vaccination is part of all national childhood immunization programmes. Reimbursement policies vary greatly between countries, depending on government or social health insurance. Thirteen countries have reimbursement policies in place for HBV treatment.
Financing for HCV treatment, including DAAs, is established in 11 countries of areas: Australia, Brunei Darussalam, Hong Kong SAR (China), Japan, Macao SAR (China), Malaysia, Mongolia, New Zealand, Republic of Korea, Singapore and Viet Nam as of February 2019. Coverage eligibility in these countries varies from specific population groups to universal access. Financing policies for health coverage among high-income countries include fixed co-payments and exemption rules according to different criteria such as age and income level. Several cities and provinces in China have begun to include the costs of DAA-based treatment for HCV under health insurance and social welfare coverage. Brunei Darussalam provides universal health care coverage, including for HCV treatment, and is making a transition to the use of DAAs as the standard of care for HCV. HCV treatment is fully reimbursed for all Australian permanent residents by Medicare, the publicly funded universal health care system, regardless of degree of fibrosis, current injecting drug use or previous treatment history. Reforms of prescribing regulations in 2016 all owed expansion of services through general practitioners and other non-specialist clinicians. This has resulted in rapid expansion of DAA treatment to over 40 000 people. By December 2016, general practitioners were writing about one third of DAA prescriptions in Australia. This was followed by further reforms allowing nurse practitioners to prescribe DAAs in 2017.
Like Australia, Japan has facilitated screening and treatment of hepatitis by general practitioners, which has allowed expanded patient access to treatment. Primary care physicians work with health centres and specialized hospitals as part of the treatment strategy. China, Malaysia, Mongolia and the Philippines are also beginning to upskill primary care providers, who are closest to the community, to provide hepatitis testing and treatment services.
Mobilizing resources for action
Many countries have a basic health insurance benefit package in place, and free HBV vaccination is part of all national childhood immunization programmes. Reimbursement policies vary greatly between countries, depending on government or social health insurance. Thirteen countries have reimbursement policies in place for HBV treatment.
Financing for HCV treatment, including DAAs, is established in 11 countries of areas: Australia, Brunei Darussalam, Hong Kong SAR (China), Japan, Macao SAR (China), Malaysia, Mongolia, New Zealand, Republic of Korea, Singapore and Viet Nam as of February 2019. Coverage eligibility in these countries varies from specific population groups to universal access. Financing policies for health coverage among high-income countries include fixed co-payments and exemption rules according to different criteria such as age and income level. Several cities and provinces in China have begun to include the costs of DAA-based treatment for HCV under health insurance and social welfare coverage. Brunei Darussalam provides universal health care coverage, including for HCV treatment, and is making a transition to the use of DAAs as the standard of care for HCV. HCV treatment is fully reimbursed for all Australian permanent residents by Medicare, the publicly funded universal health care system, regardless of degree of fibrosis, current injecting drug use or previous treatment history. Reforms of prescribing regulations in 2016 all owed expansion of services through general practitioners and other non-specialist clinicians. This has resulted in rapid expansion of DAA treatment to over 40 000 people. By December 2016, general practitioners were writing about one third of DAA prescriptions in Australia. This was followed by further reforms allowing nurse practitioners to prescribe DAAs in 2017.
Like Australia, Japan has facilitated screening and treatment of hepatitis by general practitioners, which has allowed expanded patient access to treatment. Primary care physicians work with health centres and specialized hospitals as part of the treatment strategy. China, Malaysia, Mongolia and the Philippines are also beginning to upskill primary care providers, who are closest to the community, to provide hepatitis testing and treatment services.

/59748.tmb-300v.png?sfvrsn=9885965b_2)


