Priority Area 4: Stopping transmission
Hepatitis B immunization
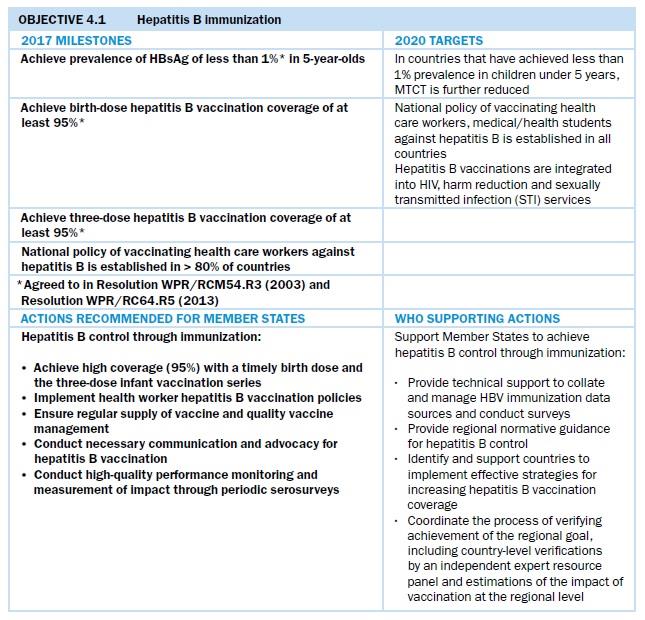
High national and regional coverage of HBV vaccination of all infants, including timely administration of a birth dose of monovalent HBV vaccine within 24 hours of delivery, remains the cornerstone of strategies to reduce vertical and horizontal transmission of HBV. All countries and areas in the Western Pacific Region have policies for universal administration of the birth dose of hepatitis B vaccine, with the exception of Japan and New Zealand. These two countries selectively administer the birth dose to children of mothers who are HBsAg-positive and to mothers with unknown HBsAg status. In May 2016, a regional study found that the Western Pacific Region’s prevalence of HBsAg among children born in 2012 was 0.93%[1]. This indicates that the 2017 target of reducing HBsAg seroprevalence among children aged 5 years and above to less than 1% (WPR/RC64.R5) has been met. Immunization programmes in this Region have averted over 7 million deaths and 37 million chronic HBV cases that would have occurred among children born between 1990 and 2014, had these HBV vaccination programmes not been established. This signifies a tremendous public health achievement for the Region. As of December 2018, 21 countries and areas in the Western Pacific had been verified by the Regional Expert Resource Panel on Hepatitis B Control through Immunization as having achieved the less than 1% HBsAg prevalence goal among children aged 5 years and above. One country was under review for verification. Two countries have serosurveys planned; serosurvey results are pending in another country; and three additional countries have serosurvey evidence of less than 1% prevalence, but to date this achievement has not been verified. Five additional countries require programme improvements (see Fig. 11).
__________
[1] Wiesen, E., S. Diorditsa, and X. Li, Progress towards hepatitis B prevention through vaccination in the Western Pacific, 1990-2014. Vaccine, 2016. 34(25): p.2855-2862

Strategies for increasing coverage of the timely hepatitis B birth dose
Data from the 2017 Joint Reporting Form indicate that 20 countries had achieved HBV birth dose coverage of at least 95%, and 18 countries and areas had achieved HBV third-dose vaccination coverage of at least 95%. Fig. 12 shows a strong correlation between birth-dose coverage and institutional delivery rates. Strengthened efforts to increase health facility deliveries, particularly in those countries with low levels of institutional delivery, are needed to facilitate timely delivery of the HBV birth dose.

Institutional delivery data not available for: Niue and areas in the Western Pacific Region under the responsibility of other Member States (including Hong Kong SAR (China), Macao SAR (China), French Polynesia, New Caledonia, Wallis and Futuna, Pitcairn Islands, American Samoa, Guam, the Commonwealth of the Northern Mariana Islands and Tokelau).
In 2016, Solomon Islands conducted a nationally representative HBV serosurvey, with results showing a high prevalence of over 3% in children of at least 5 years of age. With hundreds of islands and 35% of health facilities without any cold chain capacity, Solomon Islands was the sixth country in the Region to show that using HBV birth dose out of cold chain (OCC) could effectively and safely help break the cycle of vertical and horizontal HBV transmission. During the six-month OCC pilot study, timely birth dose coverage among facility births increased from 30% to 68% and among home births from 4% to 24%.
Solomon Islands was awarded a grant in 2017 by Gavi, the Vaccine Alliance, to equip all health facilities with cold chain capacity by 2020. Hence the Ministry of Health is forming a partnership with WHO, United Nations Children’s Fund (UNICEF) and the US Centers for Disease Control and Prevention (CDC) to expand its successful pilot study to two provinces for all health facilities that lack cold chain and for home births. This joint collaborative OCC scaling-up project represents an interim measure for health facilities that lack cold chain capacity by enabling health-care providers to provide a timely OCC birth dose, while awaiting adequate cold chain capacity. Lessons learnt from this scaling-up activity are being keenly followed by other countries considering offering birth dose OCC to remote communities and hard-to-reach areas where health facilities or home outreach are limited.
Elimination of mother-to-child transmission of viral hepatitis
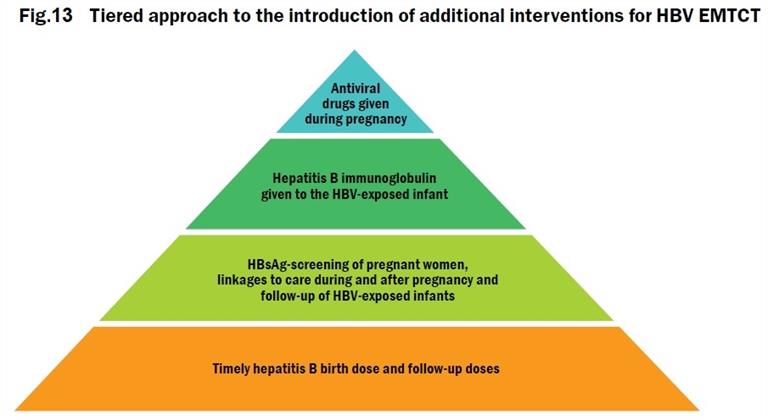
The Regional Framework for the Triple Elimination of mother-to-child transmissions of HIV, Hepatitis B and Syphilis in Asia and the Pacific, 2018–2030 was endorsed during the Regional Committee session in October 2017. This Framework proposes an integrated and coordinated approach to triple elimination – emphasizing the principle of mother newborn- and-child-centred care and a human-rights-based approach for every child, mother, her partner and their family. It also presents potential new interventions for EMTCT of HBV, building upon successful vaccination programmes to achieve 0.1% HBsAg prevalence or less among children by 2030 (Fig. 13). Countries, WHO and other stakeholders will need to work together to achieve these milestones. Strong commitment of governments, as well as cross-programme coordination and collaborations are the keys to success.
The impact target for elimination of hepatitis B set in the Regional Framework for Triple Elimination aligns with the global target of achieving a 90% reduction in new cases of chronic viral hepatitis (equivalent to 0.1% HBsAg prevalence among children). For more information, see the baseline report for triple EMTCT
A joint meeting of WHO and the National Health Commission of China was held in December 2017 to discuss HBV EMTCT in the context of triple elimination of HIV, syphilis and hepatitis B. The consultation brought together WHO, development partners, programme implementers, policy-makers, clinicians and researchers in China to review the feasibility of implementation. The meeting particularly considered the experience in use of antiviral drugs, post-vaccination serologic testing (PVST) among infants for their outcomes, plus pilot experiences of HBV EMTCT in several provinces. It also reviewed modelling results for the additional interventions to reduce MTCT of HBV towards achieving the WHO 2030 targets of 0.1% HBV seroprevalence among 5-year-old children. The meeting also discussed potential measurement and validation methods for HBV EMTCT, given the rich data and implementation experience in China. Since 2010, the Government of China has incorporated a nationwide integrated prevention of MTCT (iPMTCT) programme for HIV, syphilis and HBV in its existing maternal and child health-care system. This was built upon the national programme for PMTCT of HIV established in 2003. Since 2015, US$ 206 million has been invested in the programme, allowing expansion of geographical coverage from 2010 to 2013. The number of pregnant women attending antenatal clinics with iPMTCT services increased from 5.5 million to 13.1 million, resulting in 12.7 million pregnant women tested for HBV in 2013. iPMTCT is fully implemented through the maternal and child health-care system at county, township and village levels. Infected women are immediately enrolled for iPMTCT programme services as part of their routine antenatal, postnatal and children’s care. HBV EMTCT services, however, constitute a new policy direction that was discussed.
The Informal Consultation on Validation of Elimination of Mother-to-Child Transmission of HIV, Hepatitis B and Syphilis: Developing the Method for Validating Hepatitis B Virus Elimination was held in Putrajaya, Malaysia in February 2018. Participants discussed potential approaches for validation of EMTCT of HBV in Malaysia, and for providing input to the development of WHO guidance on EMTCT of HBV. Proposed indicators and methods for validation of EMTCT of HBV were developed. The cost and feasibility of different approaches and funding sources for national validation efforts were discussed. Finally, recommendations were developed for national guidance on process and criteria for validation of EMTCT of HBV.
Other countries are also moving towards triple elimination. Routine HBsAg screening for pregnant women is recommended by 16 countries. Cambodia developed a national strategic plan for PMTCT of HIV, syphilis and HBV from 2016 to 2020, launching its roadmap for EMTCT of HIV and syphilis on 30 November 2018. Viet Nam has also developed a national action plan for EMTCT of HIV, HBV and syphilis for 2018–2030. Malaysia is planning to expand universal HBsAg screening for pregnant women from high-risk women in Sabah to additional states. Mongolia introduced the use of antivirals for HBV-infected pregnant women with high viral load in 2017. Specific to the epidemiological context of Mongolia, testing among pregnant women included HCV and HDV. Testing among pregnant women infected with HBV is under consideration.
WHO global guidance specific to HBV is needed on interventions beyond vaccination and approaches for validation to support the goals of EMTCT. This work is in progress, and updated recommendations on the use of maternal antiviral medicines for HBV EMTCT are expected to be developed by early 2020. Meanwhile, the WHO Western Pacific Regional Office will work with WHO headquarters, countries, experts and other partners to develop an operational document on HBV EMTCT to support implementation. This will provide interim technical information and references as Member States progress towards HBV MTCT.
Global evidence shows that MTCT of hepatitis C is the primary route of transmission among children. There are large data gaps globally and, in the Region, including in the epidemiology and care of HCV among pregnant women, MTCT and long-term outcomes in their children
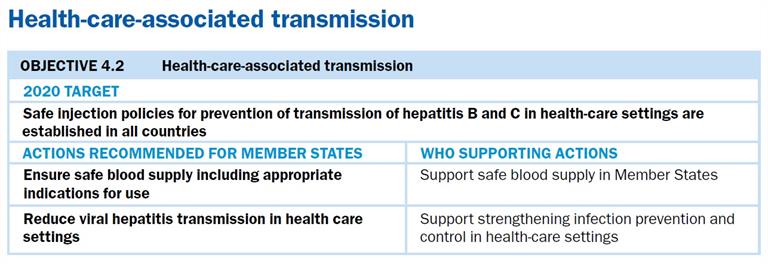
Nineteen Member States in the Region have reported having a national policy on vaccinating health-care workers against HBV (Fig. 14). A study conducted in China investigating the self-reported HBV vaccination coverage and status among 3104 health-care workers (HCWs) showed that only 60% had completed the three-dose vaccination schedule. Among those who had never received any HBV vaccination, only 30% intended to be vaccinated. Reasons for not receiving or for refusing vaccination included lack of free vaccinations, time off work to be vaccinated, lack of awareness of the need for vaccination, as well as “forgot to complete the follow-up vaccinations”[2]. The study indicated that education and national HBV vaccine policies targeting HCWs were needed, especially among older HCWs. The authors concluded that a well-managed and free national HBV vaccination policy for HCWs should be prioritized to improve HBV vaccine coverage in this high-risk occupational group. Other countries are following suit, designing research to address viral hepatitis among HCWs. For example, Cambodia and Mongolia are developing studies to assess the burden of disease in that population and to advocate for health-care vaccination. Findings will be used to help formulate a national policy specifically for HCWs. The results of these studies are vital, as they can help inform policy development in other countries across the Region.
As well, continued vigilance on infection control and universal precautions is critical. Outbreaks of infection of HIV and hepatitis C have occurred in several countries in the region due to oversights and breaks in infection control practices.
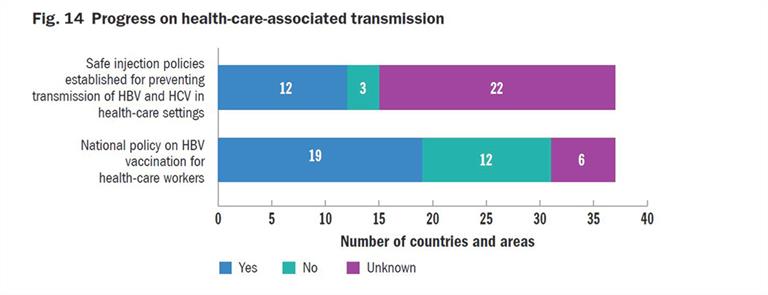
Fifteen countries in the Region have a national blood policy, and 13 have a multiyear strategic plan for blood safety (Fig. 15). Nine countries also reported having a policy of testing all blood for HCV with NAT in addition to serological testing. Twenty out of 25 countries in the Western Pacific are able to test 100% of the blood collected for HBV, but only 17 have 100% coverage for HCV.
____________
[2] Yuan, Q., et al., Hepatitis B vaccination coverage among health care workers in China. PloS one, 2019. 14(5): p. e0216598.
Harm reduction measures

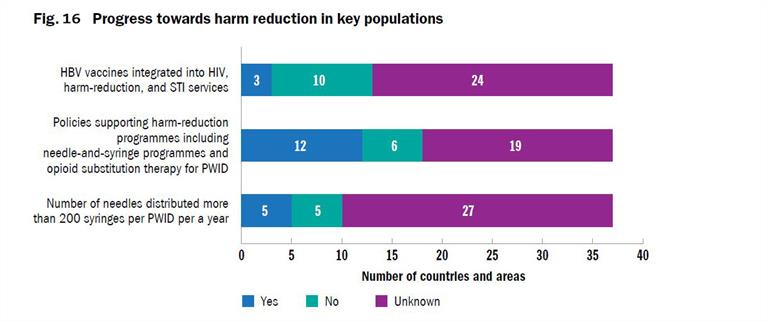
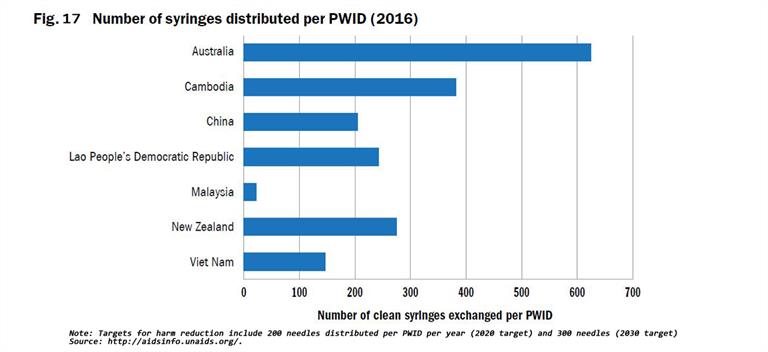
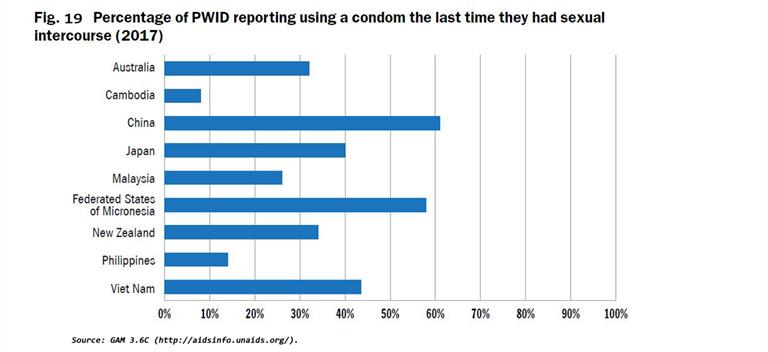
WHO, United Nations Office on Drugs and Crime (UNODC) and Joint United Nations Programme on HIV/AIDS (UNAIDS) have developed indicators with indicative targets for countries to use to monitor performance of harm reduction programmes that focus on opioid substitution therapy (OST) and needle and syringe exchange programmes (NSP) coverage and availability[3]. Full harm reduction interventions such as NSP, OST, condom use, and safe injection practices have been shown to reduce the transmission of viral hepatitis (Figs. 16 - 19). Ensuring high coverage of harm reduction interventions is associated with a reduction in viral hepatitis incidence. Combining OST and NSP is associated with a 74% reduction in new HCV infections among PWID[4].
Coverage of at least 200 sterile needles and syringes per PWID per year by 2020, and 300 by 2030, has been recommended by WHO as the minimum level needed to minimize harm. Currently, only a few countries report this number through the Global Aids Monitoring (GAM) System, and the GAM programme provides only limited data on the PWID who can access a comprehensive package of harm reduction services including: sterile injecting equipment, OST and condom use. There is a lack of data on PWID who cannot or do not access harm reduction services.
[3] World Health Organization, UNODC, and UNAIDS, WHO, UNODC, UNAIDS technical guide for countries to set targets for universal access to HIV prevention, treatment and care for injecting drug users: 2012 revision. 2013, World Health Organization: Geneva.
[4] Platt, L, et al., Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane Review and meta-analysis. Addiction, 2018. 113(3):p.545-563.